
The bond order of $He_2^ + $ molecule ion is:
A.$1$
B.$2$
C.$\dfrac{1}{2}$
D.$\dfrac{1}{4}$
Answer
552k+ views
Hint:For finding out the bond order you have to make the molecular orbital diagram for that species using MOT theory where each orbital combines with another orbital and gives the same that is two molecular orbital. The one out of which has higher energy and the other orbital will have lesser energy, fill them with electrons and then find out the difference divided by two.
Complete step-by-step answer:From MOT which is molecular orbital theory, it says that whenever the two orbitals combine like overlaps they form new molecular orbitals. These molecular orbitals are bonding and antibonding molecular orbitals one is having low energy while the other one is having high energy. This theory gives advantage if we want to calculate the bond order or we want to compare between the bond strengths etc.
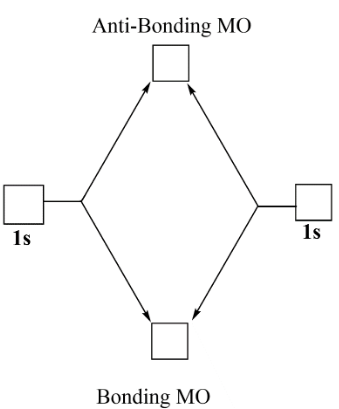
Now as we are talking about helium ion which is $He_2^ + $ so here as two helium atoms are combining and forming bond there is a positive charge over the molecule so this means that there are a total of $3$ electrons to be filled in the molecular orbitals.
$No\,of\,electrons\, = \,2(2)\, - 1$
This $ - 1$ is for the positive charge on molecules as cation. Now, filling the electrons in the molecular orbitals we get,
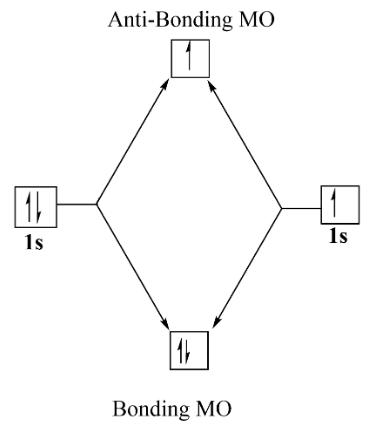
Now applying the formula for bond order which is, $Bond\,order\, = \,\dfrac{1}{2}\,\left( {{N_B} - {N_A}} \right)$
Where ${N_B}$ is the number of electrons in the bonding orbital and ${N_A}$ is electrons in the antibonding orbital. Putting the values of electrons in formula we get, $Bond\,order\, = \,\dfrac{1}{2}\,\left( {2 - 1} \right) = \,\dfrac{1}{2}$
The correct option is C.
Note:When the bond order is high the bond is difficult to break so a very high amount of energy is needed for the cleavage. Thus bond strength is high for molecules having high bond order, look for $C = C\, > \,C - C$ here in this case as olefins has the bond order of two and alkanes are having bond order one which is less, thus bond strength is also low for them.
Complete step-by-step answer:From MOT which is molecular orbital theory, it says that whenever the two orbitals combine like overlaps they form new molecular orbitals. These molecular orbitals are bonding and antibonding molecular orbitals one is having low energy while the other one is having high energy. This theory gives advantage if we want to calculate the bond order or we want to compare between the bond strengths etc.

Now as we are talking about helium ion which is $He_2^ + $ so here as two helium atoms are combining and forming bond there is a positive charge over the molecule so this means that there are a total of $3$ electrons to be filled in the molecular orbitals.
$No\,of\,electrons\, = \,2(2)\, - 1$
This $ - 1$ is for the positive charge on molecules as cation. Now, filling the electrons in the molecular orbitals we get,

Now applying the formula for bond order which is, $Bond\,order\, = \,\dfrac{1}{2}\,\left( {{N_B} - {N_A}} \right)$
Where ${N_B}$ is the number of electrons in the bonding orbital and ${N_A}$ is electrons in the antibonding orbital. Putting the values of electrons in formula we get, $Bond\,order\, = \,\dfrac{1}{2}\,\left( {2 - 1} \right) = \,\dfrac{1}{2}$
The correct option is C.
Note:When the bond order is high the bond is difficult to break so a very high amount of energy is needed for the cleavage. Thus bond strength is high for molecules having high bond order, look for $C = C\, > \,C - C$ here in this case as olefins has the bond order of two and alkanes are having bond order one which is less, thus bond strength is also low for them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




