
The bond line formula of 1-iodo-2,3-dimethyl pentane is:
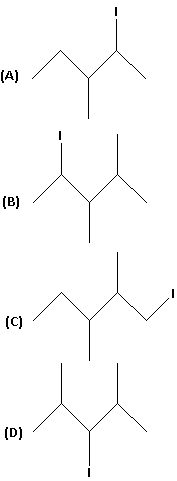

Answer
565.5k+ views
Hint:To solve this we must first draw the structure of 1-iodo-2,3-dimethyl pentane. From the structure we can then draw the bond line formula of 1-iodo-2,3-dimethyl pentane. The representation of a molecule in such a way that the bonds are represented by lines, carbon atoms are represented by line ends and intersections and atoms other than carbon and hydrogen are represented by their chemical symbols is known as a bond line formula.
Complete answer:
We are given IUPAC name of the compound as 1-iodo-2,3-dimethyl pentane.
Pentane indicates that the parent alkane is pentane and contains 5 carbon atoms.
1-iodo indicates that the iodine atom is attached to carbon number 1 of the parent alkane. The chemical symbol for iodine is ${\text{I}}$.
2,3-dimethyl indicates that two methyl groups are attached. One is attached to the carbon number 2 of the parent alkane and the other is attached at carbon number 3. The chemical formula for methyl group is ${\text{C}}{{\text{H}}_{\text{3}}}$.
Thus, the structure of 1-iodo-2,3-dimethyl pentane is as follows:
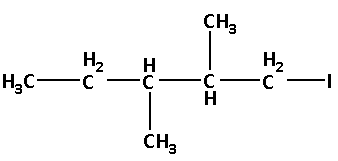
We know that the representation of a molecule in such a way that the bonds are represented by lines, carbon atoms are represented by line ends and intersections and atoms other than carbon and hydrogen are represented by their chemical symbols is known as a bond line formula. The hydrogen atoms bonded to the carbon atom are not shown in the bond line formula.
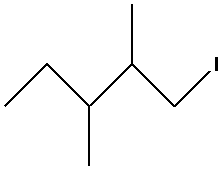
Thus, the bond line formula for 1-iodo-2,3-dimethyl pentane is as follows:
So the correct answer is option C.
Note:The bond line formula is also known as carbon skeleton formula or line segment formula. In the bond line formula, when a hydrogen atom is bonded to atoms other than carbon it is denoted by its chemical symbol. When a hydrogen atom is bonded to the carbon atom it is not shown in the bond line formula.
Complete answer:
We are given IUPAC name of the compound as 1-iodo-2,3-dimethyl pentane.
Pentane indicates that the parent alkane is pentane and contains 5 carbon atoms.
1-iodo indicates that the iodine atom is attached to carbon number 1 of the parent alkane. The chemical symbol for iodine is ${\text{I}}$.
2,3-dimethyl indicates that two methyl groups are attached. One is attached to the carbon number 2 of the parent alkane and the other is attached at carbon number 3. The chemical formula for methyl group is ${\text{C}}{{\text{H}}_{\text{3}}}$.
Thus, the structure of 1-iodo-2,3-dimethyl pentane is as follows:

We know that the representation of a molecule in such a way that the bonds are represented by lines, carbon atoms are represented by line ends and intersections and atoms other than carbon and hydrogen are represented by their chemical symbols is known as a bond line formula. The hydrogen atoms bonded to the carbon atom are not shown in the bond line formula.
Thus, the bond line formula for 1-iodo-2,3-dimethyl pentane is as follows:

So the correct answer is option C.
Note:The bond line formula is also known as carbon skeleton formula or line segment formula. In the bond line formula, when a hydrogen atom is bonded to atoms other than carbon it is denoted by its chemical symbol. When a hydrogen atom is bonded to the carbon atom it is not shown in the bond line formula.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




