
The boiling points of methane, ethane, propane and butane are given:
Compound $CH_4$ $CH_3CH_3$ $CH_3CH_2CH_3$ $CH_3CH_2CH_2CH_3$ Boiling point(K) 112 185 231 273
Which statement explains the increase in boiling point from methane to butane?
A) Closer packing of molecules results in stronger van der Waals forces.
B) More covalent bonds are present and therefore more energy is required to break the bonds.
C) More electrons in the molecules results in stronger van der Waals forces.
D) More hydrogen atoms in the molecules results in stronger hydrogen bonding.
| Compound | $CH_4$ | $CH_3CH_3$ | $CH_3CH_2CH_3$ | $CH_3CH_2CH_2CH_3$ |
| Boiling point(K) | 112 | 185 | 231 | 273 |
Answer
578.1k+ views
Hint: The temperature at which the vapour pressure of any liquid becomes equal to the atmospheric pressure on the liquid is known as the boiling point. The van der Waals forces increase from methane to butane. This is because the size of the molecule increases from methane to butane.
Complete step by step answer:
The intermolecular forces of attraction in methane, ethane, propane and butane are van der Waals forces.
The structures of methane, ethane, propane and butane are as follows:
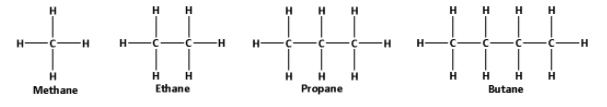
From the structures, we can say that methane is smaller than ethane which is smaller than propane which is smaller than butane. Thus, methane is the smallest and butane is the largest molecule.
As the size of the molecule increases, the number of electrons in the molecules increases. Thus, the number of electrons increases from methane to butane and as a result the strength of the van der Waals forces increases.
The van der Waals forces increase as the size of the molecule increases. Thus, the van der Waals forces are weakest in methane and strongest in butane.
Stronger the intermolecular force, higher is the boiling point because more energy will be required to break the bonds.
Thus, the statement ‘more electrons in the molecules results in stronger van der Waals forces’ explains the increase in boiling point from methane to butane.
Thus, the correct option is (C) more electrons in the molecules results in stronger van der Waals forces.
Additional Information: The melting points of the alkanes also gradually increase from methane to butane. This is because of the increase in van der Waals forces from methane to butane.
Note: Hydrogen bonding is when a hydrogen atom bonded to an electronegative atom bonds to another electronegative atom. Thus, hydrogen bonding does not occur in alkanes. All the bonds in alkanes are nonpolar bonds.
Complete step by step answer:
The intermolecular forces of attraction in methane, ethane, propane and butane are van der Waals forces.
The structures of methane, ethane, propane and butane are as follows:

From the structures, we can say that methane is smaller than ethane which is smaller than propane which is smaller than butane. Thus, methane is the smallest and butane is the largest molecule.
As the size of the molecule increases, the number of electrons in the molecules increases. Thus, the number of electrons increases from methane to butane and as a result the strength of the van der Waals forces increases.
The van der Waals forces increase as the size of the molecule increases. Thus, the van der Waals forces are weakest in methane and strongest in butane.
Stronger the intermolecular force, higher is the boiling point because more energy will be required to break the bonds.
Thus, the statement ‘more electrons in the molecules results in stronger van der Waals forces’ explains the increase in boiling point from methane to butane.
Thus, the correct option is (C) more electrons in the molecules results in stronger van der Waals forces.
Additional Information: The melting points of the alkanes also gradually increase from methane to butane. This is because of the increase in van der Waals forces from methane to butane.
Note: Hydrogen bonding is when a hydrogen atom bonded to an electronegative atom bonds to another electronegative atom. Thus, hydrogen bonding does not occur in alkanes. All the bonds in alkanes are nonpolar bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




