
The $BC{{l}_{3}}$ is a planar molecule whereas $NC{{l}_{3}}$ is pyramidal because:
(a)- Nitrogen atom is smaller than the boron atom
(b)- $BC{{l}_{3}}$ has no lone pair but $NC{{l}_{3}}$ has a lone pair of electrons
(c)- $BCl$ bond is more polar than $NCl$ bond
(d)- $NCl$ bond is more covalent than $BCl$ bond
Answer
565.2k+ views
Hint: If the central atom is joined with two atoms then the shape is expected to be linear, if the central atom is joined with 3 atoms then the shape is expected to be trigonal planar, if the central atom is joined with 4 atoms then the shape is expected to be tetrahedral and so on. But if the molecule has a lone pair of electrons then the geometry of the compound will change.
Complete Solution :
In $BC{{l}_{3}}$, boron is the central atom and it is the element of group 13 of the p-block, so it will have three valence electrons in its valence shell for the formation of bonds. In this molecule, there are three chlorine atoms and all the three valence electrons of the boron are involved in the bond formation. So, if the central atom is joined with 3 atoms then the shape is expected to be trigonal planar, therefore, its structure will be trigonal planar. The structure is given below:
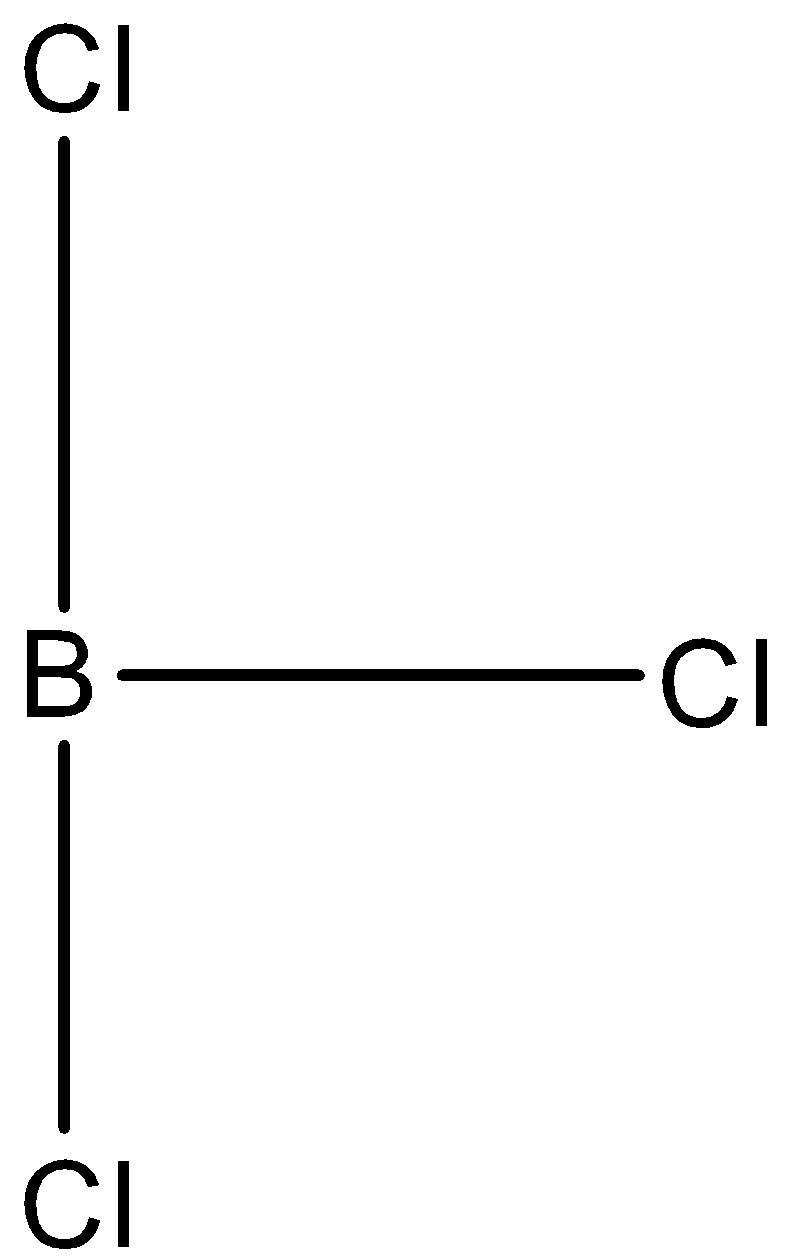
- In $NC{{l}_{3}}$, nitrogen is the central atom and it is the element of group 15 of the p-block, so it will have five valence electrons in its valence shell for the formation of bonds. In this molecule, there are three chlorine atoms, and three out of five valence electrons of the nitrogen are involved in the bond formation, and the rest two electrons will be the lone pair. Now, due to this lone of electrons, the bond pairs will come close to each other forming a pyramidal shape. The structure is given below:

Therefore, the correct answer is option (b)- $BC{{l}_{3}}$ has no lone pair but $NC{{l}_{3}}$ has a lone pair of electrons.
So, the correct answer is “Option B”.
Note: It must be noted that the repulsion between the bond pair-bond pair is less than the repulsion between the lone pair-bond pair. This will decrease the bond angle. As the number of lone pairs increases the repulsion also increases.
Complete Solution :
In $BC{{l}_{3}}$, boron is the central atom and it is the element of group 13 of the p-block, so it will have three valence electrons in its valence shell for the formation of bonds. In this molecule, there are three chlorine atoms and all the three valence electrons of the boron are involved in the bond formation. So, if the central atom is joined with 3 atoms then the shape is expected to be trigonal planar, therefore, its structure will be trigonal planar. The structure is given below:

- In $NC{{l}_{3}}$, nitrogen is the central atom and it is the element of group 15 of the p-block, so it will have five valence electrons in its valence shell for the formation of bonds. In this molecule, there are three chlorine atoms, and three out of five valence electrons of the nitrogen are involved in the bond formation, and the rest two electrons will be the lone pair. Now, due to this lone of electrons, the bond pairs will come close to each other forming a pyramidal shape. The structure is given below:

Therefore, the correct answer is option (b)- $BC{{l}_{3}}$ has no lone pair but $NC{{l}_{3}}$ has a lone pair of electrons.
So, the correct answer is “Option B”.
Note: It must be noted that the repulsion between the bond pair-bond pair is less than the repulsion between the lone pair-bond pair. This will decrease the bond angle. As the number of lone pairs increases the repulsion also increases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




