
The atomicity of phosphorus is X and the P-P-P bond angle is Y. What are X and Y?
Answer
601.5k+ views
Hint: To solve this question, we need to know the regular tetrahedron structure of phosphorus. As we see in the figure below, we have 6 P-P single bonds and also 4 lone pairs of electrons in this molecule. The bond length of P-P is 2.21 A and P-P-P angle and atomicity is asked in this question.
Complete step by step solution:
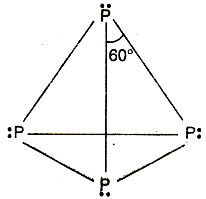
As we see in given diagram, we have
In white phosphorus ${P_4}$ ,
6 P-P single bonds
4 lone pair of electrons
Bond angle ∠P-P-P=60 degrees.
$P_4$ molecules are sometimes called as white phosphorus, in which the crystal structure of red phosphorus has a complicated network of bonding. And also, it has to be stored in water to prevent natural combustion, but red phosphorus is stable in air.
The molecular formula of white phosphorus is ${P_4}$. Four phosphorus atoms lie at the corners of the regular tetrahedron In the structure of White phosphorus. Each phosphorus atom is linked to other three phosphorus atoms by covalent bonds. The bond length of P-P is 2.21 A and PPP angle is 60 degrees.
${P_4}$ exists as tetrahedra tetratomic discrete molecule with P−P−P bond angle of 60$^\circ$,
Hence atomicity of P=4.
Note: White phosphorus is a waxy solid which burns easily and is used in chemical manufacturing and smoke munitions. When phosphorus is exposed to white may cause irritation and burns, liver, kidney, heart, lung, or bone damage, and death.
And also, it causes severely painful, partial (second degree) to full thickness (third degree) burns, which have a characteristic yellow color and garlic-like odor. burn site because Smoke may be released due to the continued burning of white phosphorus or the formation of phosphoric acid.
Complete step by step solution:

As we see in given diagram, we have
In white phosphorus ${P_4}$ ,
6 P-P single bonds
4 lone pair of electrons
Bond angle ∠P-P-P=60 degrees.
$P_4$ molecules are sometimes called as white phosphorus, in which the crystal structure of red phosphorus has a complicated network of bonding. And also, it has to be stored in water to prevent natural combustion, but red phosphorus is stable in air.
The molecular formula of white phosphorus is ${P_4}$. Four phosphorus atoms lie at the corners of the regular tetrahedron In the structure of White phosphorus. Each phosphorus atom is linked to other three phosphorus atoms by covalent bonds. The bond length of P-P is 2.21 A and PPP angle is 60 degrees.
${P_4}$ exists as tetrahedra tetratomic discrete molecule with P−P−P bond angle of 60$^\circ$,
Hence atomicity of P=4.
Note: White phosphorus is a waxy solid which burns easily and is used in chemical manufacturing and smoke munitions. When phosphorus is exposed to white may cause irritation and burns, liver, kidney, heart, lung, or bone damage, and death.
And also, it causes severely painful, partial (second degree) to full thickness (third degree) burns, which have a characteristic yellow color and garlic-like odor. burn site because Smoke may be released due to the continued burning of white phosphorus or the formation of phosphoric acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




