
The anion of acetylacetone (acac) forms $[Co{(acac)_3}]$ chelates with $C{O_3}^ + $ .The rings of the chelates are:
A.Three membered
B.Five membered
C.Four membered
D.Six membered
Answer
589.5k+ views
Hint: Basically, the Lewis base which donates two lone pairs of electrons to the central metal atom is known as bidentate ligand or chelating ligands. Further, the complex which contains chelating ligands is known as chelated.
Complete step by step answer:
Generally, a ligand is an ion or molecule which donates a pair of electrons to the central metal atom or ion to form a coordination complex. These can be anions, cations and neutral molecules. We can also say that a ligand is an ion or molecule which binds to the central metal atom to form a coordination entity or complex compounds. Now, we have different types of ligands such as mono dentate ligand, bidentate ligand and tri dentate ligand.
Now, the Lewis base which donates two lone pairs of electrons to the central metal atom is known as bidentate ligands. They are often called chelating ligands and the complex which contains chelating ligands is known as chelates. Moreover, the complexes containing chelate rings are more stable than the complexes without rings. This is known as chelate effect. So, for the given compound, we will have six membered chelate rings i.e. the Ligand acetyl acetone forms six membered chelate ring in the complex $[Co{(acac)_3}]$ which is as shown:
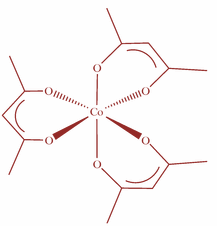
Hence, option D is correct.
Note: The ligands with more than one potential donor atoms are known as ambidentate ligands. For example, thiocyanate ion i.e. ${(NCS)^ - }$ . It can bind to the central metal atom or ion with either nitrogen or Sulphur atoms. Moreover, we have one more type of ligand known as bridging ligands. This is the type of ligand which is bound to more than one metal atom.
Complete step by step answer:
Generally, a ligand is an ion or molecule which donates a pair of electrons to the central metal atom or ion to form a coordination complex. These can be anions, cations and neutral molecules. We can also say that a ligand is an ion or molecule which binds to the central metal atom to form a coordination entity or complex compounds. Now, we have different types of ligands such as mono dentate ligand, bidentate ligand and tri dentate ligand.
Now, the Lewis base which donates two lone pairs of electrons to the central metal atom is known as bidentate ligands. They are often called chelating ligands and the complex which contains chelating ligands is known as chelates. Moreover, the complexes containing chelate rings are more stable than the complexes without rings. This is known as chelate effect. So, for the given compound, we will have six membered chelate rings i.e. the Ligand acetyl acetone forms six membered chelate ring in the complex $[Co{(acac)_3}]$ which is as shown:

Hence, option D is correct.
Note: The ligands with more than one potential donor atoms are known as ambidentate ligands. For example, thiocyanate ion i.e. ${(NCS)^ - }$ . It can bind to the central metal atom or ion with either nitrogen or Sulphur atoms. Moreover, we have one more type of ligand known as bridging ligands. This is the type of ligand which is bound to more than one metal atom.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




