
How many tetrahedral angles are there in a methane molecule?
Answer
538.2k+ views
Hint: As we know that methane molecules have a tetrahedral geometry, therefore we will try to attempt this question by drawing the tetrahedral structure of methane and then count the number of angles. Every angle between the bonds in tetrahedral geometry are equal and measured to be${{109.5}^{\circ }}$.
Complete answer:
As we know that in tetrahedral, tetra signifies four and hedral relates to a face of a solid which makes the conclusion of “having four faces”. This shape is found where four bonds all are attached to one central atom, with no lone electron pairs.
-According to the VSEPR (valence shell electron pair repulsion) theory, the bond angles between the electron bonds are measured to be${{109.5}^{\circ }}$. An example of a tetrahedral molecule is methane ($C{{H}_{4}}$ ).
-The four equivalent bonds (C-H) point in four geometrically equivalent directions in three dimensions which can be corresponded to the four corners of a tetrahedron as hydrogen, centered on the carbon atom. The shape of methane is shown below:-
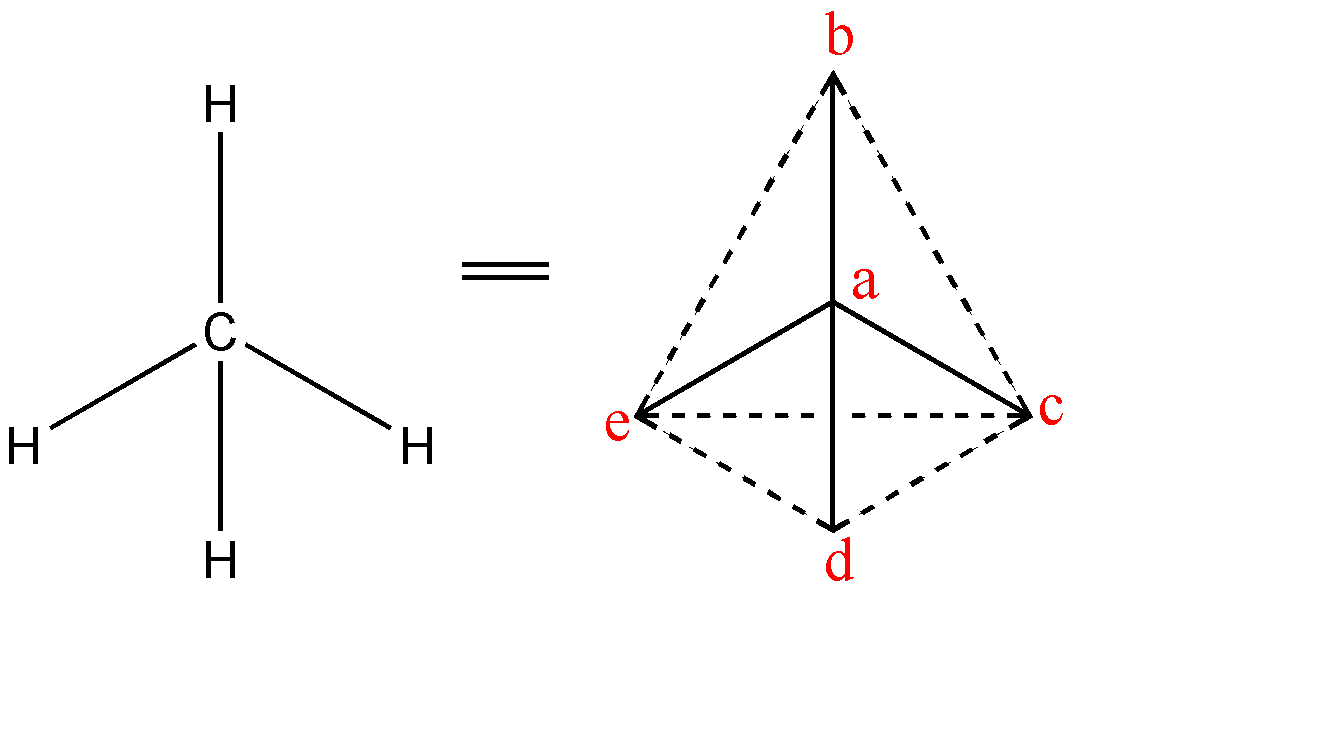
The angles between the bonds are as follows:-
1.$\angle cad$
2. $\angle cae$
3. $\angle cab$
4. $\angle dae$
5. $\angle dab$
6. $\angle eab$
-Above are all the possible angles that can be in a tetrahedral shape. Therefore, total number of tetrahedral angles in a methane molecule = 6
Note:
While counting the bond angles, always prefer to make shapes over geometry of the given molecule as bond angle is always between bonds, not with lone pairs.
-Geometry gives us the overall arrangement of a molecule including bonds and lone pairs whereas shape only considers real bonds.
Complete answer:
As we know that in tetrahedral, tetra signifies four and hedral relates to a face of a solid which makes the conclusion of “having four faces”. This shape is found where four bonds all are attached to one central atom, with no lone electron pairs.
-According to the VSEPR (valence shell electron pair repulsion) theory, the bond angles between the electron bonds are measured to be${{109.5}^{\circ }}$. An example of a tetrahedral molecule is methane ($C{{H}_{4}}$ ).
-The four equivalent bonds (C-H) point in four geometrically equivalent directions in three dimensions which can be corresponded to the four corners of a tetrahedron as hydrogen, centered on the carbon atom. The shape of methane is shown below:-

The angles between the bonds are as follows:-
1.$\angle cad$
2. $\angle cae$
3. $\angle cab$
4. $\angle dae$
5. $\angle dab$
6. $\angle eab$
-Above are all the possible angles that can be in a tetrahedral shape. Therefore, total number of tetrahedral angles in a methane molecule = 6
Note:
While counting the bond angles, always prefer to make shapes over geometry of the given molecule as bond angle is always between bonds, not with lone pairs.
-Geometry gives us the overall arrangement of a molecule including bonds and lone pairs whereas shape only considers real bonds.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




