
Tertiary butyl methyl ether on treatment with $ HI $ forms:
(A) Isobutane and methyl iodide
(B) Isobutanol and methanol
(C) Tertiary butyl iodide and methyl iodide
(D) tertiary butyl iodide and methanol
Answer
514.5k+ views
Hint: The action of hydrogen iodide on any ether is to cleave the ether into two fragments. One of the two fragments contains an oxygen atom and the other does not. The fragment will contain oxygen or not depending upon the mechanism through which the reaction happens.
Complete answer:
Hydrogen iodide reacts with ether to give a nucleophilic substitution reaction in which one alkyl group of the ether gets attached to the iodide molecule and becomes an alkyl halide and the other alkyl group that is connected to oxygen form a bond with hydrogen (coming from the dissociation of hydrogen iodide) to form the corresponding alcohol.
The mechanism of the reaction depends on the type of alkyl groups present in the ether molecule. If the alkyl are primary or secondary in nature, then a bimolecular substitution $ S{N^2} $ reaction takes place and the iodide ion (that acts as the incoming nucleophile) reacts from a less hindered position.
If the ether contains a tertiary alkyl group then the mechanism that is followed is unimolecular $ S{N^1} $ substitution. The driving force of the reaction is formation of a tertiary carbocation which gets attacked by the iodide nucleophile.
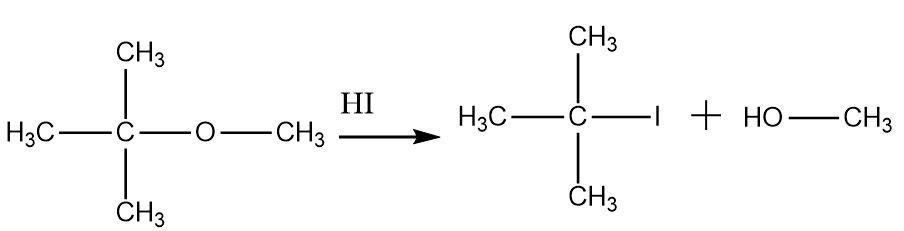
Thus Tertiary butyl methyl ether on treatment with $ HI $ forms tertiary butyl iodide and methanol, option (d) is correct.
Note:
A tertiary carbocation formation is preferred in all organic reactions due to its high stability. The tertiary carbocation is stabilized by the phenomenon of hyperconjugation. The greater number of $ \alpha - hydrogens $ present in a tertiary carbocation increases the number of hyperconjugative structures and hence the stability gets enhanced.
Complete answer:
Hydrogen iodide reacts with ether to give a nucleophilic substitution reaction in which one alkyl group of the ether gets attached to the iodide molecule and becomes an alkyl halide and the other alkyl group that is connected to oxygen form a bond with hydrogen (coming from the dissociation of hydrogen iodide) to form the corresponding alcohol.
The mechanism of the reaction depends on the type of alkyl groups present in the ether molecule. If the alkyl are primary or secondary in nature, then a bimolecular substitution $ S{N^2} $ reaction takes place and the iodide ion (that acts as the incoming nucleophile) reacts from a less hindered position.
If the ether contains a tertiary alkyl group then the mechanism that is followed is unimolecular $ S{N^1} $ substitution. The driving force of the reaction is formation of a tertiary carbocation which gets attacked by the iodide nucleophile.

Thus Tertiary butyl methyl ether on treatment with $ HI $ forms tertiary butyl iodide and methanol, option (d) is correct.
Note:
A tertiary carbocation formation is preferred in all organic reactions due to its high stability. The tertiary carbocation is stabilized by the phenomenon of hyperconjugation. The greater number of $ \alpha - hydrogens $ present in a tertiary carbocation increases the number of hyperconjugative structures and hence the stability gets enhanced.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




