
Tertiary alkyl halide is:
A.$C{H_3} - CH(C{H_3}) - Br$
B.$C{H_3} - C{(C{H_3})_2} - C{H_2}Br$
C.$C{H_3} - C{H_2} - C{H_2} - Br$
D.$C{H_3} - C{(C{H_3})_2} - Br$
Answer
559.2k+ views
Hint:Primary, secondary and tertiary are distinctions of hydrocarbons based on relative position of other carbon atoms. Find the number of methyl groups attached to halogen containing carbon atoms and find the tertiary carbon atom.
Complete answer:
Alkyl halides are hydrocarbons containing one or more halogen groups. Alkyl halides as to be discussed here are of three types which are primary alkyl halide, secondary alkyl halide and tertiary alkyl halide.
This distinction is based on the number of carbon atoms attached to the carbon atom which contains the halogen group. The halogen we have here is bromine. If the halogen containing carbon atom is attached to only one carbon atom it is called primary alkyl halide. If the halogen containing carbon atom is attached to two carbon atoms it is called secondary alkyl halide. If the halogen containing carbon atom is attached to three carbon atoms it is called tertiary alkyl halide.
Now we have to look into the structure of each compound to see which halogen containing carbon atom is attached to three other carbon atoms, that will be the tertiary alkyl halide.
If we look at the last structure as the halogen containing carbon looks to be most populated,
Now as we can see, bromine containing carbon atoms is attached to three other methyl groups. So that carbon is said to be a tertiary carbon atom and the compound is tertiary alkyl halide.
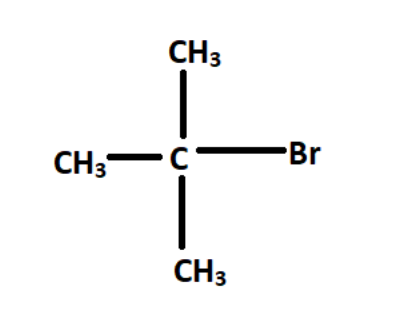
So, option D is the correct answer.
Note:
For a tertiary hydrocarbon, there must be at least four carbon atoms in the hydrocarbon. There can be no tertiary three degree carbon atom in a linear hydrocarbon chain, the hydrocarbon must be branched.
Complete answer:
Alkyl halides are hydrocarbons containing one or more halogen groups. Alkyl halides as to be discussed here are of three types which are primary alkyl halide, secondary alkyl halide and tertiary alkyl halide.
This distinction is based on the number of carbon atoms attached to the carbon atom which contains the halogen group. The halogen we have here is bromine. If the halogen containing carbon atom is attached to only one carbon atom it is called primary alkyl halide. If the halogen containing carbon atom is attached to two carbon atoms it is called secondary alkyl halide. If the halogen containing carbon atom is attached to three carbon atoms it is called tertiary alkyl halide.
Now we have to look into the structure of each compound to see which halogen containing carbon atom is attached to three other carbon atoms, that will be the tertiary alkyl halide.
If we look at the last structure as the halogen containing carbon looks to be most populated,
Now as we can see, bromine containing carbon atoms is attached to three other methyl groups. So that carbon is said to be a tertiary carbon atom and the compound is tertiary alkyl halide.

So, option D is the correct answer.
Note:
For a tertiary hydrocarbon, there must be at least four carbon atoms in the hydrocarbon. There can be no tertiary three degree carbon atom in a linear hydrocarbon chain, the hydrocarbon must be branched.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




