
Teflon is an example of polymer which is/are -
A.Polyamide
B.Addition polymer
C.Polyester
D.Formaldehyde resin.
Answer
567.9k+ views
Hint:It is known, a polymer is a substance or material which consists of a very large number of molecules, or macromolecules, and is composed of many repeating subunits. Basically, they are materials made of long, repeating chains of molecules.
Complete step by step answer:
Polymers , based on their polymerisation, can be classified mainly into two categories -
Addition polymerisation, which is basically when a polymer is, is formed by simple linking of monomers without the co-generation of other products.
Condensation polymerisation, which is formed by the step-growth polymerization in which monomers or oligomers react with each other to form larger structural units while releasing smaller molecules as a by - product such as water or methanol, mainly water.
So as we know, Teflon is formed from the monomer tetrafluoroethene by its repeated addition. It is also a homopolymer i.e. consisting of a single monomer.
And hence option B is the correct answer.
Additional information
The structure of the monomer of Teflon is -
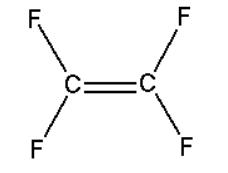
The carbons are $s{p_2}$ hybridized, and they share a double bond while the two fluorine atoms present are bonded to each carbon, and their geometry around the carbon atoms is of trigonal planar.
Note:
Polytetrafluoroethylene or Teflon is best known for its use in making the coating of non-stick frying pans and other cookware, as it is known to be hydrophobic and possesses fairly a very high heat resistance.
Complete step by step answer:
Polymers , based on their polymerisation, can be classified mainly into two categories -
Addition polymerisation, which is basically when a polymer is, is formed by simple linking of monomers without the co-generation of other products.
Condensation polymerisation, which is formed by the step-growth polymerization in which monomers or oligomers react with each other to form larger structural units while releasing smaller molecules as a by - product such as water or methanol, mainly water.
So as we know, Teflon is formed from the monomer tetrafluoroethene by its repeated addition. It is also a homopolymer i.e. consisting of a single monomer.
And hence option B is the correct answer.
Additional information
The structure of the monomer of Teflon is -

The carbons are $s{p_2}$ hybridized, and they share a double bond while the two fluorine atoms present are bonded to each carbon, and their geometry around the carbon atoms is of trigonal planar.
Note:
Polytetrafluoroethylene or Teflon is best known for its use in making the coating of non-stick frying pans and other cookware, as it is known to be hydrophobic and possesses fairly a very high heat resistance.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




