
How to synthesize p-nitrobenzoic acid and m-nitrobenzoic acid starting from benzene?
Answer
561.3k+ views
Hint:The p-nitrobenzoic acid and m-nitrobenzoic acid is prepared from benzene by different steps which involves Friedel Craft alkylation reaction, nitration reaction and oxidation reaction.
Complete step by step answer:For the preparation of p-nitrobenzoic acid and m-nitrobenzoic acid from benzene, different steps are involved.
The reaction for the preparation of p-nitrobenzoic acid from benzene is shown below.
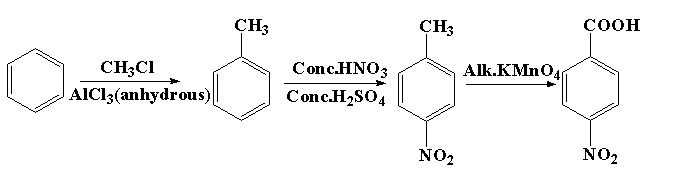
In this reaction, first benzene is reacted with methyl chloride in presence of anhydrous aluminum trichloride to form toluene. This reaction is known as Friedal craft alkylation reaction where the alkyl group is attached to the benzene ring. After this nitration reaction takes place. In nitration the toluene is reacted with a mixture of concentration nitric acid and sulphuric acid to form p-nitrotoluene. In the next process, p-nitrotoluene is treated with alkaline potassium permanganate to form p-nitrobenzoic acid. The potassium permanganate acts as the oxidizing agent which oxidizes the methyl group to the carboxylic acid group.
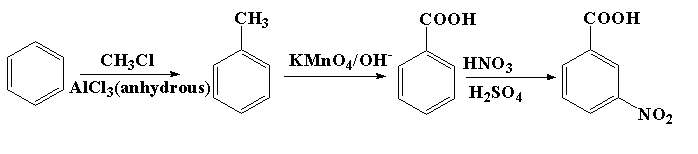
In this reaction, benzene reacts with methyl chloride in presence of anhydrous aluminium trichloride to form toluene. The toluene is then reacted with alkaline potassium permanganate to form benzoic acid. The benzoic acid then undergoes a nitration reaction in presence of nitric acid and sulphuric acid to form m-nitrobenzoic acid.
Note:
Don’t get confused over Friedel Craft alkylation and Friedel Craft acylation reaction as both are electrophilic aromatic substitution reactions. In the acylation reaction acyl group is attached to the benzene ring. The other oxidizing agents are potassium chromate, hydrogen peroxide.
Complete step by step answer:For the preparation of p-nitrobenzoic acid and m-nitrobenzoic acid from benzene, different steps are involved.
The reaction for the preparation of p-nitrobenzoic acid from benzene is shown below.

In this reaction, first benzene is reacted with methyl chloride in presence of anhydrous aluminum trichloride to form toluene. This reaction is known as Friedal craft alkylation reaction where the alkyl group is attached to the benzene ring. After this nitration reaction takes place. In nitration the toluene is reacted with a mixture of concentration nitric acid and sulphuric acid to form p-nitrotoluene. In the next process, p-nitrotoluene is treated with alkaline potassium permanganate to form p-nitrobenzoic acid. The potassium permanganate acts as the oxidizing agent which oxidizes the methyl group to the carboxylic acid group.

In this reaction, benzene reacts with methyl chloride in presence of anhydrous aluminium trichloride to form toluene. The toluene is then reacted with alkaline potassium permanganate to form benzoic acid. The benzoic acid then undergoes a nitration reaction in presence of nitric acid and sulphuric acid to form m-nitrobenzoic acid.
Note:
Don’t get confused over Friedel Craft alkylation and Friedel Craft acylation reaction as both are electrophilic aromatic substitution reactions. In the acylation reaction acyl group is attached to the benzene ring. The other oxidizing agents are potassium chromate, hydrogen peroxide.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




