
How will you synthesize chloroprene from acetylene?
Answer
512.4k+ views
Hint: Acetylene is an organic compound in which there are two carbon atoms and there is a triple bond present. Chloroprene is a compound in which there are four carbon atoms and at the 2nd position, the chlorine atom is present. To convert acetylene to chloroprene, there will be two steps.
Complete answer:
For the conversion of acetylene to chloroprene, there will be two-step reactions.
Acetylene is an organic compound in which there are two carbon atoms and there is a triple bond present. The formula of acetylene is $HC\equiv CH$. It is also known as ethyne.
Chloroprene is a compound in which there are four carbon atoms and at the 2nd position, the chlorine atom is present. In the first position and third position, there are double bonds. The structure of chloroprene is given below:
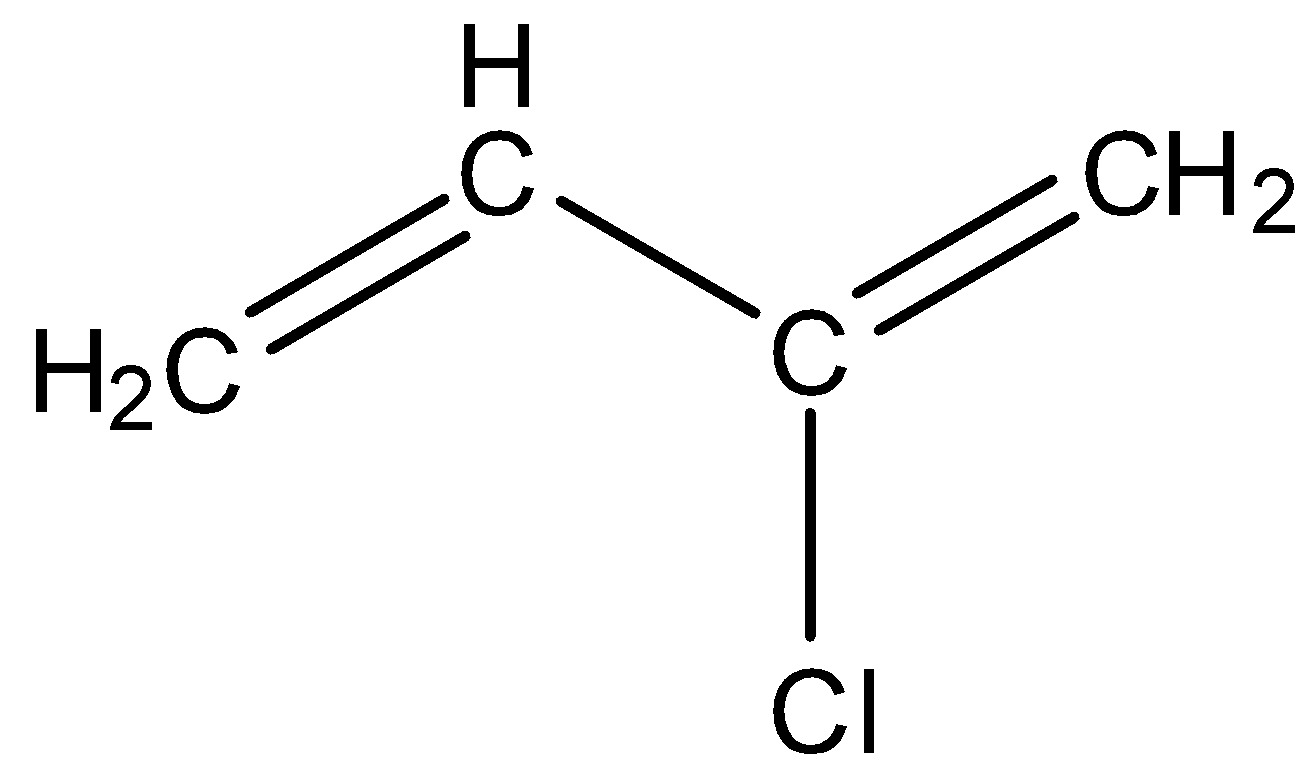
In the first step, there will be dimerization acetylene. So, 2 molecules of acetylene will react with each other to form divinylacetylene. The reaction is given below:
$2HC\equiv CH\to {{H}_{2}}C=CH-C\equiv CH$
In the second step, the divinyl acetylene is treated with hydrochloric acid (HCl) in the presence of carbon tetrachloride. So, the hydrochloric acid will attack the triple bond of divinylacetylene. The chlorine atom will attack the carbon atom having a lesser number of hydrogen atoms and the hydrogen atom will attack the other carbon atom. Therefore, the product formed will be chloroprene. The reaction is given below:
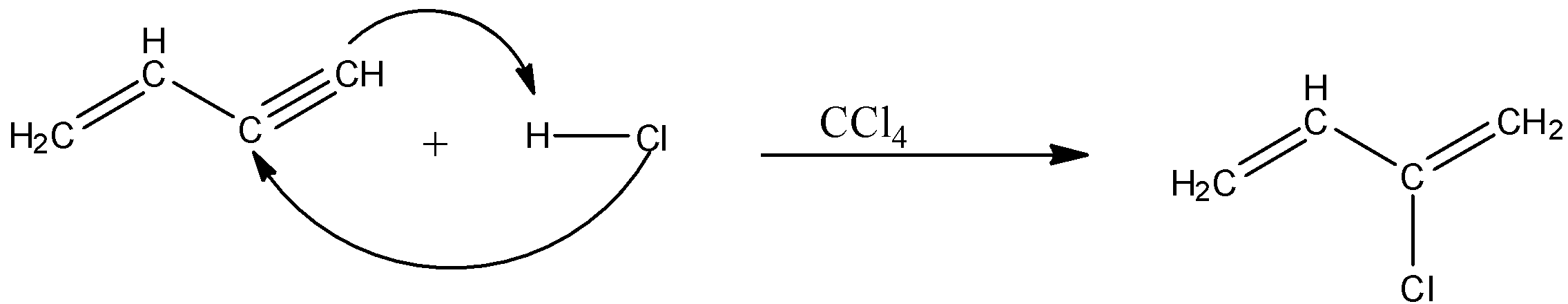
So, the acetylene is converted into chloroprene.
Note:
In the above process, the temperature of the second reaction is ${{60}^{\circ }}C$. In the traditional method, in the second step, with the hydrochloric acid solution, CuCl and $N{{H}_{4}}Cl$ is used and this takes place at ${{80}^{\circ }}C$ temperature.
Complete answer:
For the conversion of acetylene to chloroprene, there will be two-step reactions.
Acetylene is an organic compound in which there are two carbon atoms and there is a triple bond present. The formula of acetylene is $HC\equiv CH$. It is also known as ethyne.
Chloroprene is a compound in which there are four carbon atoms and at the 2nd position, the chlorine atom is present. In the first position and third position, there are double bonds. The structure of chloroprene is given below:

In the first step, there will be dimerization acetylene. So, 2 molecules of acetylene will react with each other to form divinylacetylene. The reaction is given below:
$2HC\equiv CH\to {{H}_{2}}C=CH-C\equiv CH$
In the second step, the divinyl acetylene is treated with hydrochloric acid (HCl) in the presence of carbon tetrachloride. So, the hydrochloric acid will attack the triple bond of divinylacetylene. The chlorine atom will attack the carbon atom having a lesser number of hydrogen atoms and the hydrogen atom will attack the other carbon atom. Therefore, the product formed will be chloroprene. The reaction is given below:

So, the acetylene is converted into chloroprene.
Note:
In the above process, the temperature of the second reaction is ${{60}^{\circ }}C$. In the traditional method, in the second step, with the hydrochloric acid solution, CuCl and $N{{H}_{4}}Cl$ is used and this takes place at ${{80}^{\circ }}C$ temperature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




