
How is sulphur dioxide represented by VSEPR? What is the shape of the molecule?
Answer
487.5k+ views
Hint: The valence shell electron pair repulsion hypothesis is a chemical model that predicts a molecule's shape based on the number of electron pairs around its core atoms. The assumption of VSEPR is that the valence electron pairs around an atom resist each other and, as a result, would organise themselves in a way that minimises this repulsion. This reduces the energy of the molecule and enhances its stability, determining the molecular shape.
Complete answer:
The chemical molecule $S{{O}_{2}}$ stands for sulphur dioxide. It's a poisonous gas that gives out the odour of burned matches. It is generated as a by-product of copper mining and the combustion of sulfur-bearing fossil fuels and is released naturally by volcanic activity. The odour of sulphur dioxide is similar to that of nitric acid.
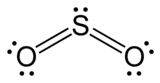
The type of hybridization that occurs in sulphur dioxide is \[s{{p}^{2}}\]. To do so, we'll start with the sulphur atom, which will be the centre atom. This core atom is linked with two oxygen atoms during the production of \[S{{O}_{2}}\], and their structure is \[O=S=O\]. There is one sigma and one pi connection created between sulphur and the two oxygen atoms in terms of bonding. One lone pair can also be accommodated in the atom.
Sulphur's ground state has six electrons in the outermost shell, and the first two shells are likewise entirely filled. The $3p$ portal has four electrons, while the 3s orbital has two paired electrons. It now requires four unpaired electrons to establish four bonds (with oxygen). This causes the excited state in sulphur to form, in which one \[3{{p}_{x}}\] electron jumps to the 3d orbital. There will be one unpaired electron in one 3d orbital and three in 3p orbitals when this happens. The electrons that make up the sigma bonds and the lone pair, on the other hand, are at different energy levels. When hybridization occurs, the stable state is reached.
Two 3p orbitals and one 3s orbital are hybridised throughout the process. There are a total of three \[s{{p}^{2}}\] hybrid orbitals forming. Two hybrid orbitals will have unpaired electrons, while the lone pair will be in one hybrid orbital. The oxygen atoms subsequently form sigma bonds with the unpaired electrons. The 3d and 3p orbitals, on the other hand, stay unchanged and play a role in the creation of pi bonds.

Note:
The molecular geometry of \[S{{O}_{2}}\] is thought to be V-shaped or twisted. Sulphur dioxide's electron geometry, on the other hand, is in the shape of a trigonal planar. The three pairs of bonding electrons are angled at 119 degrees. There are two double pairs bonded together, as well as one lone pair, giving it a bent form.
Complete answer:
The chemical molecule $S{{O}_{2}}$ stands for sulphur dioxide. It's a poisonous gas that gives out the odour of burned matches. It is generated as a by-product of copper mining and the combustion of sulfur-bearing fossil fuels and is released naturally by volcanic activity. The odour of sulphur dioxide is similar to that of nitric acid.
The type of hybridization that occurs in sulphur dioxide is \[s{{p}^{2}}\]. To do so, we'll start with the sulphur atom, which will be the centre atom. This core atom is linked with two oxygen atoms during the production of \[S{{O}_{2}}\], and their structure is \[O=S=O\]. There is one sigma and one pi connection created between sulphur and the two oxygen atoms in terms of bonding. One lone pair can also be accommodated in the atom.
Sulphur's ground state has six electrons in the outermost shell, and the first two shells are likewise entirely filled. The $3p$ portal has four electrons, while the 3s orbital has two paired electrons. It now requires four unpaired electrons to establish four bonds (with oxygen). This causes the excited state in sulphur to form, in which one \[3{{p}_{x}}\] electron jumps to the 3d orbital. There will be one unpaired electron in one 3d orbital and three in 3p orbitals when this happens. The electrons that make up the sigma bonds and the lone pair, on the other hand, are at different energy levels. When hybridization occurs, the stable state is reached.
Two 3p orbitals and one 3s orbital are hybridised throughout the process. There are a total of three \[s{{p}^{2}}\] hybrid orbitals forming. Two hybrid orbitals will have unpaired electrons, while the lone pair will be in one hybrid orbital. The oxygen atoms subsequently form sigma bonds with the unpaired electrons. The 3d and 3p orbitals, on the other hand, stay unchanged and play a role in the creation of pi bonds.


Note:
The molecular geometry of \[S{{O}_{2}}\] is thought to be V-shaped or twisted. Sulphur dioxide's electron geometry, on the other hand, is in the shape of a trigonal planar. The three pairs of bonding electrons are angled at 119 degrees. There are two double pairs bonded together, as well as one lone pair, giving it a bent form.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




