
What is sublimation? Perform an experiment to demonstrate the sublimation of camphor or ammonium chloride. Draw a labelled diagram.
Answer
503.4k+ views
Hint: Sublimation is the process of a substance transitioning directly from a solid to a gas state without passing through a liquid stage. Sublimation is an endothermic process that occurs when a substance's triple point on its phase diagram, which corresponds to the lowest pressure at which the substance can exist as a liquid, is reached. Deposition or desublimation is the reversal of sublimation, in which a substance transitions from a gas to a solid state.
Complete answer:
A solid-to-gas transition (sublimation) followed by a gas-to-solid transition has also been referred to as sublimation (deposition). While vaporisation from a liquid to a gas occurs as evaporation from the surface if it occurs below the boiling point of the liquid and as boiling with the formation of bubbles in the interior of the liquid if it occurs at the boiling point, the solid-to-gas transition occurs as sublimation from the surface.
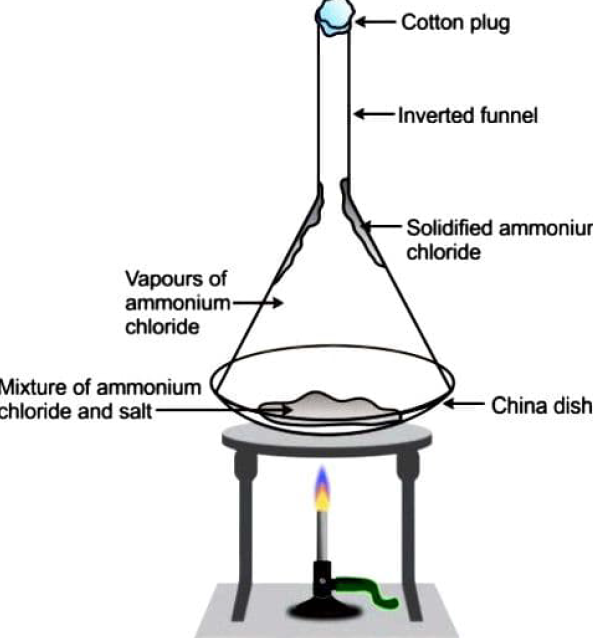
We know that when ammonium chloride is heated, it transforms from a solid to a gas. The sublimation procedure is used to separate a combination containing a sublimable volatile component from a non-sublimable contaminant (in this case, salt). It's a phase transition in which stuff travels straight from solid to gaseous form, or vapour, without going through the more typical liquid phase in between.
Note:
Most chemical compounds and elements have three distinct states at different temperatures under normal pressures. The shift from solid to gaseous state in many circumstances necessitates an intermediate liquid state. The pressure being discussed is the partial pressure of the substance, not the entire system's total (e.g. atmospheric) pressure.
Complete answer:
A solid-to-gas transition (sublimation) followed by a gas-to-solid transition has also been referred to as sublimation (deposition). While vaporisation from a liquid to a gas occurs as evaporation from the surface if it occurs below the boiling point of the liquid and as boiling with the formation of bubbles in the interior of the liquid if it occurs at the boiling point, the solid-to-gas transition occurs as sublimation from the surface.

We know that when ammonium chloride is heated, it transforms from a solid to a gas. The sublimation procedure is used to separate a combination containing a sublimable volatile component from a non-sublimable contaminant (in this case, salt). It's a phase transition in which stuff travels straight from solid to gaseous form, or vapour, without going through the more typical liquid phase in between.
Note:
Most chemical compounds and elements have three distinct states at different temperatures under normal pressures. The shift from solid to gaseous state in many circumstances necessitates an intermediate liquid state. The pressure being discussed is the partial pressure of the substance, not the entire system's total (e.g. atmospheric) pressure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




