
Structure of Propanone is:
(A) $C{{H}_{3}}-C{{H}_{2}}-C{{H}_{3}}$
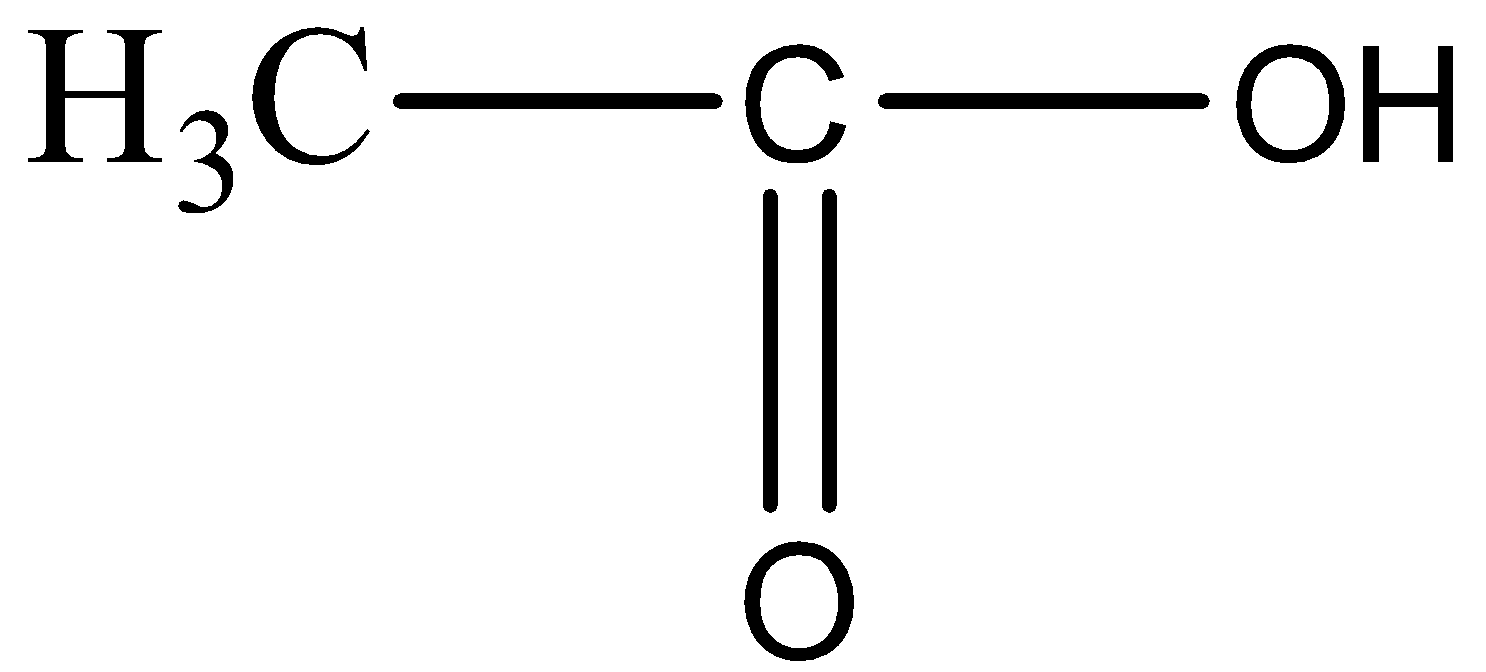
(B)
(C) $C{{H}_{3}}-O-{{C}_{3}}{{H}_{7}}$
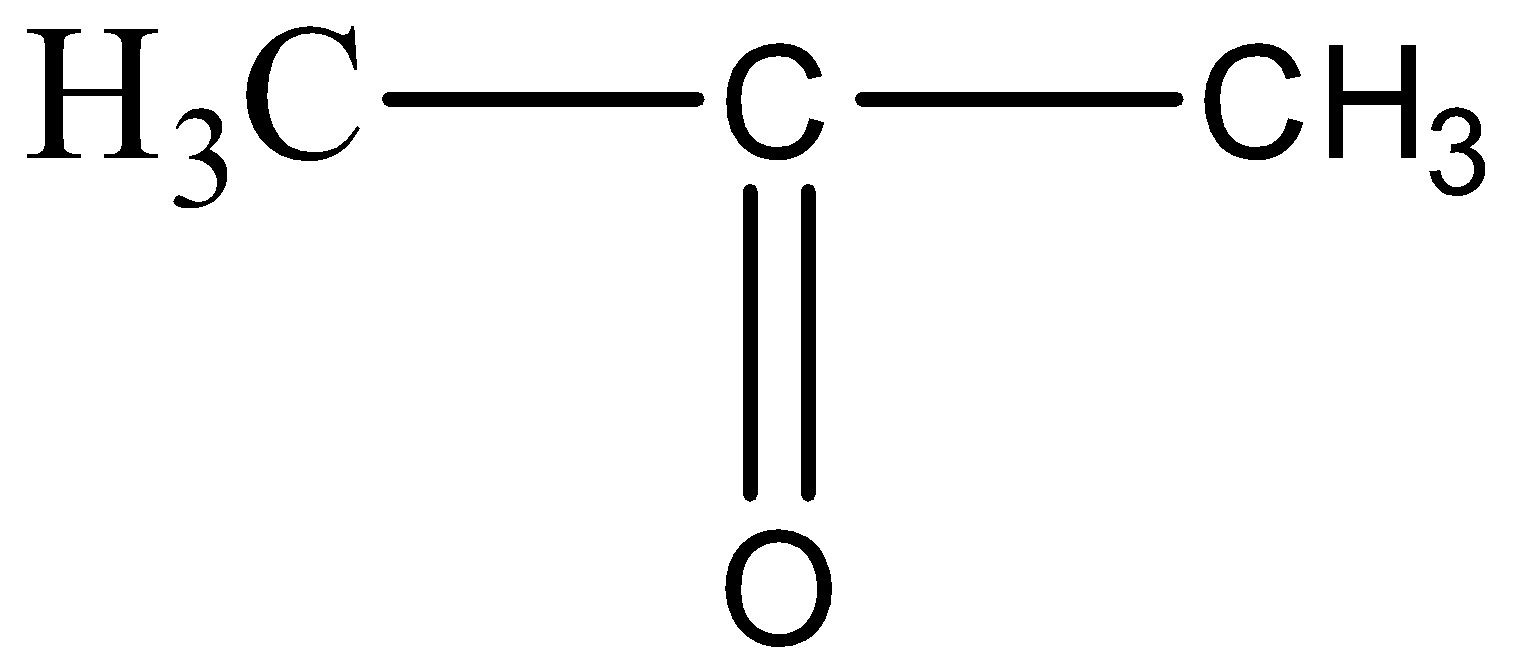
(D)
(E) $C{{H}_{3}}-C{{H}_{2}}-N{{H}_{2}}$


Answer
588.6k+ views
Hint: Propanone is an organic compound, which has the formula of ${{\text{(C}{{\text{H}}_{\text{3}}}\text{)}}_{\text{2}}}\text{CO}$. It is also known as Acetone. On the basis of the formula, we can predict the structure of Propanone.
Complete step by step answer:
Let’s analyse the name Propanone.
- Here, the suffix –one is used. So, it suggests the presence of the ketone functional group.
- Now, in the IUPAC nomenclature, we use the name of the parent alkyl chain in the compound then use necessary prefixes and suffixes. Here, no prefix is given that means there are no substituted groups present on the parent alkyl chain. The word ‘prop’ suggests the presence of the parent alkyl chain that has a total of three carbon atoms in a straight line.
- Now, for a compound to be a ketone, it needs to have two alkyl groups attached to the carbonyl carbon. The only way we can arrange the structure of Propanone is to set two methyl groups bonded with a carbonyl group. Let’s check other options also.
For option A,
$C{{H}_{3}}-C{{H}_{2}}-C{{H}_{3}}$
We can see that there is no carbon – oxygen double bond present on the compound. So, this cannot be the answer.
For option B,
In the compound given above there are two carbon atoms and OH functional group, which does not go with the formula of Propanone. So, this cannot be the answer.
For option C,
$C{{H}_{3}}-O-{{C}_{3}}{{H}_{7}}$
The compound that is given above, shows us the absence of carbon – oxygen double bond and at the same time there are two carbon atoms on the main carbon chain. So, this cannot be the correct answer.
For option D,
The structure resembles the formula of Propanone, since there are three carbon atoms and one carbon – oxygen double bond.
For option E,
$C{{H}_{3}}-C{{H}_{2}}-N{{H}_{2}}$
The structure has the absence of carbon – oxygen double bond and two numbers of carbon atoms. So, this cannot be the answer. So, the correct answer is “Option D”.
Note: Remember that there is only one possibility of the place of the carbonyl group in the propanone molecule so that the resulting molecule becomes a ketone. There should be a prior knowledge about the compound that is mentioned in the question. Propanone is the smallest and the simplest ketone.
Complete step by step answer:
Let’s analyse the name Propanone.
- Here, the suffix –one is used. So, it suggests the presence of the ketone functional group.
- Now, in the IUPAC nomenclature, we use the name of the parent alkyl chain in the compound then use necessary prefixes and suffixes. Here, no prefix is given that means there are no substituted groups present on the parent alkyl chain. The word ‘prop’ suggests the presence of the parent alkyl chain that has a total of three carbon atoms in a straight line.
- Now, for a compound to be a ketone, it needs to have two alkyl groups attached to the carbonyl carbon. The only way we can arrange the structure of Propanone is to set two methyl groups bonded with a carbonyl group. Let’s check other options also.
For option A,
$C{{H}_{3}}-C{{H}_{2}}-C{{H}_{3}}$
We can see that there is no carbon – oxygen double bond present on the compound. So, this cannot be the answer.
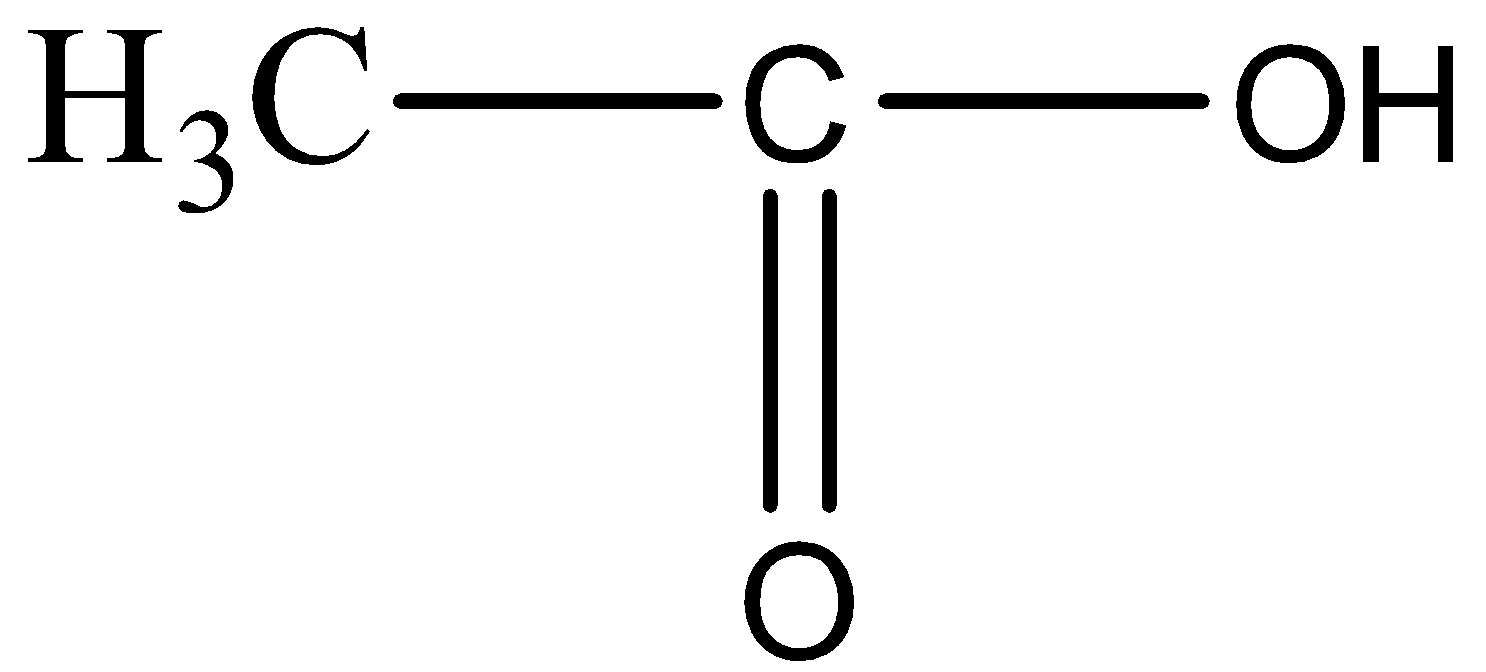
For option B,

In the compound given above there are two carbon atoms and OH functional group, which does not go with the formula of Propanone. So, this cannot be the answer.
For option C,
$C{{H}_{3}}-O-{{C}_{3}}{{H}_{7}}$
The compound that is given above, shows us the absence of carbon – oxygen double bond and at the same time there are two carbon atoms on the main carbon chain. So, this cannot be the correct answer.
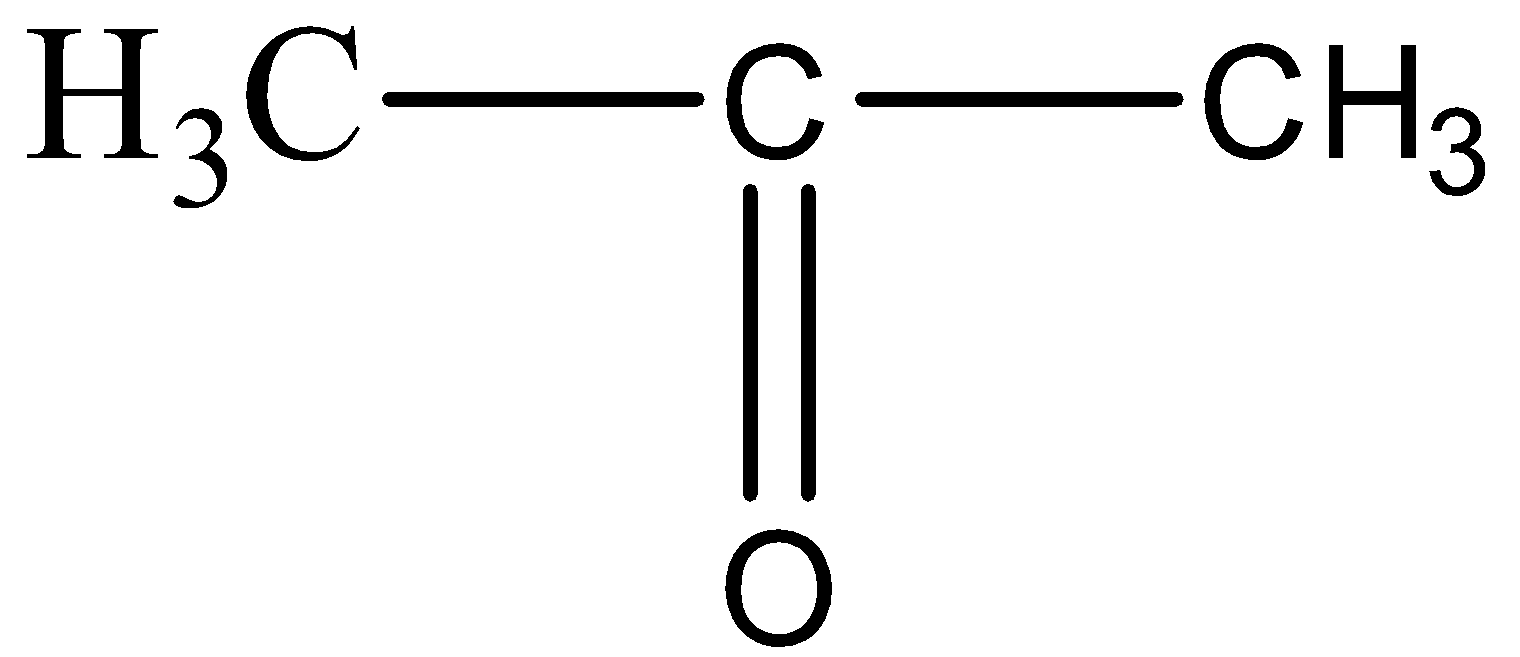
For option D,

The structure resembles the formula of Propanone, since there are three carbon atoms and one carbon – oxygen double bond.
For option E,
$C{{H}_{3}}-C{{H}_{2}}-N{{H}_{2}}$
The structure has the absence of carbon – oxygen double bond and two numbers of carbon atoms. So, this cannot be the answer. So, the correct answer is “Option D”.
Note: Remember that there is only one possibility of the place of the carbonyl group in the propanone molecule so that the resulting molecule becomes a ketone. There should be a prior knowledge about the compound that is mentioned in the question. Propanone is the smallest and the simplest ketone.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




