
What is the structure of NaCl?
(A) BCC
(B) FCC
(C) Interpenetrating FCC
(D) None of these
Answer
559.8k+ views
Hint: $ \text{NaCl} $ has a cubic unit cell. It is best thought of as an FCC array of anions with an inter penetrating FCC cation lattice. The cell looks the same whether you start with anions or cation on the corners. Each ion is $ \text{6} $ coordinate and has a local quadrilateral geometry.
Complete Step by step solution
The unit cell of NaCl consists of $ \text{N}{{\text{a}}^{+}} $ ions and $ \text{C}{{\text{l}}^{-}} $ ions. There are four types of site-unique central position, face site, edge sites and corner site which are used to determine the number of $ \text{N}{{\text{a}}^{+}} $ and $ \text{C}{{\text{l}}^{-}} $ ions in the unit cell of NaCl. When counting the number of ions, a corner site would be shared by $ 7 $ other unit cells. Therefore, $ 1 $ corner would be $ \dfrac{1}{8} $ of an ion. For a face site, it is shared by other unit cells and for an edge site, the ion is shared by $ 3 $ other unit cells. NaCl is a FCC unit cell which has four cations and four anions. This can be shown by counting the number of ions and multiplying them in relation to their position.
$ \text{N}{{\text{a}}^{+}}:\text{ }{{\text{1}}_{center}}\text{ + 1}{{\text{2}}_{edge}}\times \dfrac{1}{4}=4 $
$ \text{C}{{\text{l}}^{-}}\text{ }:\text{ }{{\text{4}}_{face}}\times \dfrac{1}{2}+{{8}_{corner}}\times \dfrac{1}{8}=4 $
The crystal structure of $ \text{NaCl} $ is as shown below:
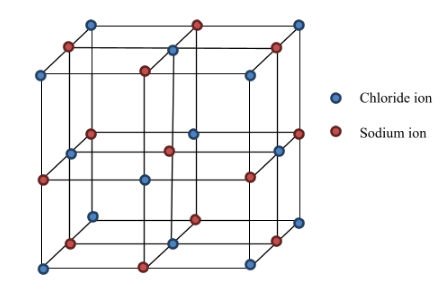
In NaCl, each ion is surrounded by six ions of two opposite charges as expected on electrostatic grounds.
Note
In addition to this, NaCl also known as salt or halite. A halite is an ionic compound with the chemical formula NaCl , representing a $ 1:1 $ ratio of sodium and chloride ions. Sodium chloride salt occurs in oceans and sea waters. It is also formed as Rock salt. About $ 1% $ to $ 5% $ of seawater is made of NaCl It is a crystalline solid, which has white color. In aqueous form, it is called saline water.
Complete Step by step solution
The unit cell of NaCl consists of $ \text{N}{{\text{a}}^{+}} $ ions and $ \text{C}{{\text{l}}^{-}} $ ions. There are four types of site-unique central position, face site, edge sites and corner site which are used to determine the number of $ \text{N}{{\text{a}}^{+}} $ and $ \text{C}{{\text{l}}^{-}} $ ions in the unit cell of NaCl. When counting the number of ions, a corner site would be shared by $ 7 $ other unit cells. Therefore, $ 1 $ corner would be $ \dfrac{1}{8} $ of an ion. For a face site, it is shared by other unit cells and for an edge site, the ion is shared by $ 3 $ other unit cells. NaCl is a FCC unit cell which has four cations and four anions. This can be shown by counting the number of ions and multiplying them in relation to their position.
$ \text{N}{{\text{a}}^{+}}:\text{ }{{\text{1}}_{center}}\text{ + 1}{{\text{2}}_{edge}}\times \dfrac{1}{4}=4 $
$ \text{C}{{\text{l}}^{-}}\text{ }:\text{ }{{\text{4}}_{face}}\times \dfrac{1}{2}+{{8}_{corner}}\times \dfrac{1}{8}=4 $
The crystal structure of $ \text{NaCl} $ is as shown below:

In NaCl, each ion is surrounded by six ions of two opposite charges as expected on electrostatic grounds.
Note
In addition to this, NaCl also known as salt or halite. A halite is an ionic compound with the chemical formula NaCl , representing a $ 1:1 $ ratio of sodium and chloride ions. Sodium chloride salt occurs in oceans and sea waters. It is also formed as Rock salt. About $ 1% $ to $ 5% $ of seawater is made of NaCl It is a crystalline solid, which has white color. In aqueous form, it is called saline water.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




