
What is the structure of acetal formed by reaction of ethanol with acetophenone?
Answer
519k+ views
Hint: We can say that acetal is an organic functional group that chemical formula of ${R_2}C{\left( {O{R^1}} \right)_2}$. Here, we can say that organic groups (or) hydrogen are given as R and the other organic groups are given as R. We have to know that the central carbon atom exhibits tetrahedral geometry.
Complete answer:
We have to know that acetals are given from and convertible to aldehydes or ketones and have a similar oxidation state at the central carbon, yet have considerably extraordinary substance stability and reactivity when contrasted with the practically equivalent to carbonyl compounds.
We have to know that the term ketal is here and there used to distinguish structures related to ketones (both R groups are organic segments instead of hydrogen) as opposed to aldehydes.
We can form acetal/ketal when a hemiacetal/hemiketal is reacted with alcohols. We have to know hemiacetals/hemiketals are formed when an aldehyde/ketone is treated with alcohols. Water gets eliminated.
We can write the general equation of acetal formation from aldehyde as,
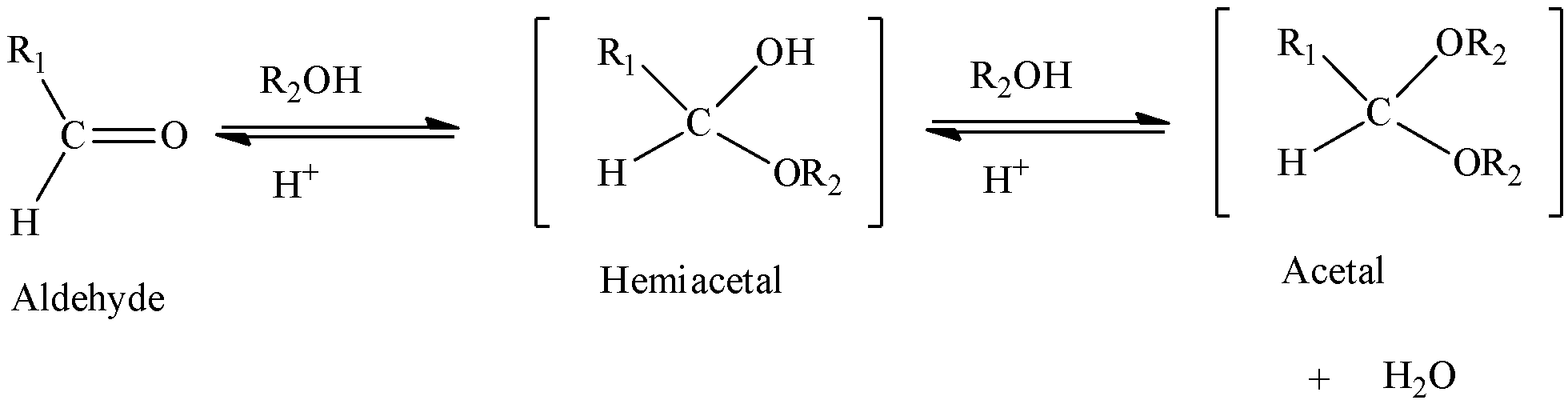
We can write the general equation of ketal formation from ketone as,
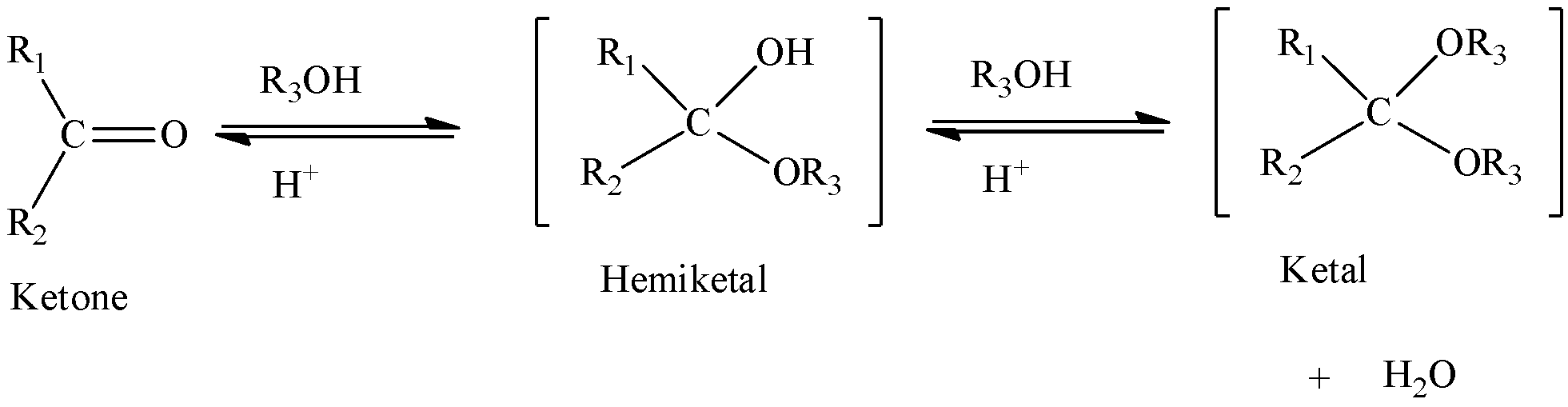
We know that ethanol is an alcohol and acetophenone is a ketone. Acetophenone contains methyl group and benzyl group that is bonded to carbonyl group present in the centre.
In this reaction, ${R_2}$ is ${C_6}{H_5}C{H_3}$ and ${R^1}$ is $C{H_2}C{H_3}$.
We can write the formation reaction of acetal from ethanol and acetophenone as,
${C_6}{H_5}COC{H_3} + 2C{H_3}C{H_2}OH\xrightarrow{{HCl}}{C_6}{H_5}C\left( {C{H_3}} \right){\left( {OC{H_2}C{H_3}} \right)_2}$
We can write the structure of acetal as ${C_6}{H_5}C\left( {C{H_3}} \right){\left( {OC{H_2}C{H_3}} \right)_2}$.
Note:
We have to know that the name of an organic reaction which involves the formation of acetal is acetalization. One should not get confused between hemiacetal and acetal. Hemiacetal contains hydroxyl group and alkoxy group, whereas acetal contains only alkoxy group. When aldehyde (or) ketone reacts with one alcohol molecule, a hemiacetal is obtained whereas when aldehyde (or) ketone reacts with two alcohol molecules, acetal is obtained.
Complete answer:
We have to know that acetals are given from and convertible to aldehydes or ketones and have a similar oxidation state at the central carbon, yet have considerably extraordinary substance stability and reactivity when contrasted with the practically equivalent to carbonyl compounds.
We have to know that the term ketal is here and there used to distinguish structures related to ketones (both R groups are organic segments instead of hydrogen) as opposed to aldehydes.
We can form acetal/ketal when a hemiacetal/hemiketal is reacted with alcohols. We have to know hemiacetals/hemiketals are formed when an aldehyde/ketone is treated with alcohols. Water gets eliminated.
We can write the general equation of acetal formation from aldehyde as,

We can write the general equation of ketal formation from ketone as,

We know that ethanol is an alcohol and acetophenone is a ketone. Acetophenone contains methyl group and benzyl group that is bonded to carbonyl group present in the centre.
In this reaction, ${R_2}$ is ${C_6}{H_5}C{H_3}$ and ${R^1}$ is $C{H_2}C{H_3}$.
We can write the formation reaction of acetal from ethanol and acetophenone as,
${C_6}{H_5}COC{H_3} + 2C{H_3}C{H_2}OH\xrightarrow{{HCl}}{C_6}{H_5}C\left( {C{H_3}} \right){\left( {OC{H_2}C{H_3}} \right)_2}$
We can write the structure of acetal as ${C_6}{H_5}C\left( {C{H_3}} \right){\left( {OC{H_2}C{H_3}} \right)_2}$.
Note:
We have to know that the name of an organic reaction which involves the formation of acetal is acetalization. One should not get confused between hemiacetal and acetal. Hemiacetal contains hydroxyl group and alkoxy group, whereas acetal contains only alkoxy group. When aldehyde (or) ketone reacts with one alcohol molecule, a hemiacetal is obtained whereas when aldehyde (or) ketone reacts with two alcohol molecules, acetal is obtained.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




