
How many structural isomers can you draw for pentane?
Answer
597k+ views
Hint: Structural isomers are those which have the same molecular formula and different bonding patterns and atomic organization. Pentane is an organic compound with 5 carbon atoms attached with single bonds. So, just see the number of arrangements formed by pentane.
Complete step by step answer:
-Pentane is a 5 carbon organic compound and since the suffix is –ane we can say that there is only single bonds (it is a saturated molecule). There are no double or triple bonds (it is not unsaturated).
-The molecular formula of Pentane is: \[{C_5}{H_{12}}\]
-The 5 carbon atoms of pentane can be arranged in following distinct forms:
n-pentane or pentane: in this first arrangement all the 5 carbon atoms are arranged one after the other to form a single chain with no branching. It is known as n-pentane and its molecular formula is \[{C_5}{H_{12}}\]. Its structural formula is:
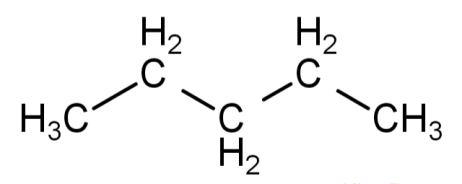
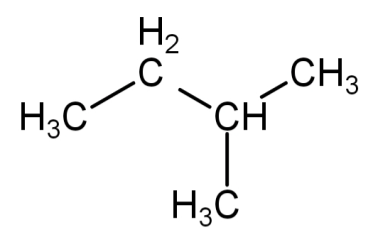
Isopentane or 2-methylbutane: in this type of arrangement 4 carbon atoms are arranged in a straight chain and the 5th carbon atom is attached to the 2nd carbon atom of the straight chain to form a branch. This is known as isopentane and the IUPAC name is 2-methylbutane. Its molecular formula is \[{C_5}{H_{12}}\].
Its structural formula is:
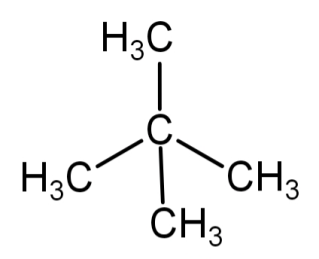
Neopentane or dimethylpropane: in this type of arrangement 3 carbon atoms are arranged one after the other to form a straight chain, while the other 2 carbon atoms are attached to the middle carbon atom of the straight chain to form 2 branches. It is known as neopentane and the IUPAC name is dimethylpropane. Its molecular formula is \[{C_5}{H_{12}}\] .
Its structural formula is:
No other patterns or types of structures are possible for pentane. So, we can say that pentane has 3 structural isomers: 1) n-pentane
2) isopentane (2-methylbutane)
3) neopentane (dimethylpropane)
Note: While naming any molecule the carbon chain should be numbered from the side where the branching is closer and not the other way round. Basically , we need to give the branch or functional group the lowest number possible. This helps us ensure that all of our isomers are distinct and not similar.
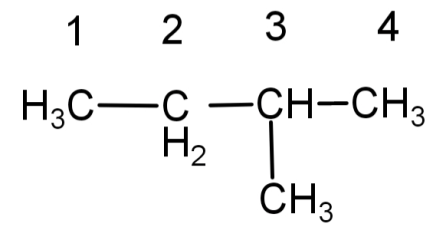
(A) Numbering done here is wrong.
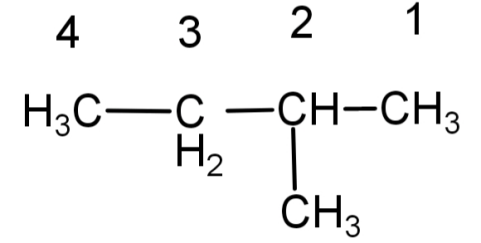
(B) Numbering done here is correct.
So, don’t confuse the above 2 isomers as different counting them as 2 isomers, they both are the same , the only difference is the numbering of the carbon atoms which is wrong in (A) and correct in (B).
Complete step by step answer:
-Pentane is a 5 carbon organic compound and since the suffix is –ane we can say that there is only single bonds (it is a saturated molecule). There are no double or triple bonds (it is not unsaturated).
-The molecular formula of Pentane is: \[{C_5}{H_{12}}\]
-The 5 carbon atoms of pentane can be arranged in following distinct forms:
n-pentane or pentane: in this first arrangement all the 5 carbon atoms are arranged one after the other to form a single chain with no branching. It is known as n-pentane and its molecular formula is \[{C_5}{H_{12}}\]. Its structural formula is:

Isopentane or 2-methylbutane: in this type of arrangement 4 carbon atoms are arranged in a straight chain and the 5th carbon atom is attached to the 2nd carbon atom of the straight chain to form a branch. This is known as isopentane and the IUPAC name is 2-methylbutane. Its molecular formula is \[{C_5}{H_{12}}\].
Its structural formula is:

Neopentane or dimethylpropane: in this type of arrangement 3 carbon atoms are arranged one after the other to form a straight chain, while the other 2 carbon atoms are attached to the middle carbon atom of the straight chain to form 2 branches. It is known as neopentane and the IUPAC name is dimethylpropane. Its molecular formula is \[{C_5}{H_{12}}\] .
Its structural formula is:

No other patterns or types of structures are possible for pentane. So, we can say that pentane has 3 structural isomers: 1) n-pentane
2) isopentane (2-methylbutane)
3) neopentane (dimethylpropane)
Note: While naming any molecule the carbon chain should be numbered from the side where the branching is closer and not the other way round. Basically , we need to give the branch or functional group the lowest number possible. This helps us ensure that all of our isomers are distinct and not similar.

(A) Numbering done here is wrong.

(B) Numbering done here is correct.
So, don’t confuse the above 2 isomers as different counting them as 2 isomers, they both are the same , the only difference is the numbering of the carbon atoms which is wrong in (A) and correct in (B).
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

The Equation xxx + 2 is Satisfied when x is Equal to Class 10 Maths

Which Country is Called "The Land of Festivals"?

What is Contraception List its four different methods class 10 biology CBSE




