
What is the structural formula of $CaC{{O}_{3}}$?
Answer
531.3k+ views
Hint: A structural formula of any compound is used to denote the shape and position and nature of bonds in that compound. Ionic compounds are formed by transfer of electrons; they are represented as their structural ions. $CaC{{O}_{3}}$ is calcium carbonate which is an ionic compound that has carbonate anion and calcium cation.
Complete answer:
When a compound is formed by the transfer of electrons from one atom to another, then it is termed as an ionic compound. Ionic compounds consist of ions cation and anion. Cations have a positive charge as they donate their valence electron to fulfill their octet, and anions gain electrons to complete their octet. The structural formula of ionic compounds is written in their respective ionic symbols as they are not bonded by electrons, but formed through electron transfer.
We have been given to give the formula for $CaC{{O}_{3}}$ which is an ionic compound. It contains calcium as a cation and carbonate ion as an anion. Calcium loses 1 electron from its valence shell to become $C{{a}^{+}}$ this electron is taken up by one carbonate anion to complete their octet and forms $C{{O}_{3}}^{-}$. The structural formulas of them are:
Calcium is present as $C{{a}^{+}}$ ,
While carbonate as
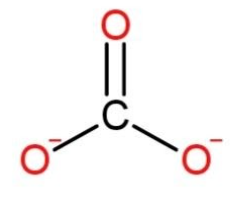
Calcium is in a form of cation as it is a monatomic ion, while carbonate being a polyatomic ion has a trigonal planar shape.
Hence the structural formula of $CaC{{O}_{3}}$ is
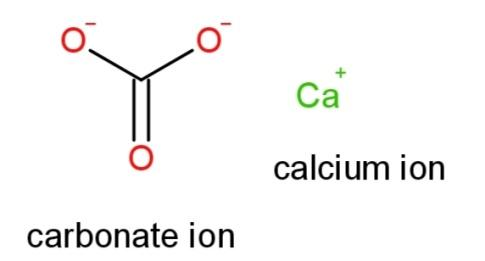
Note:
Calcium carbonate is an important ionic compound. It is formed from the alkali metal calcium which is a constituent part of bones. It is therefore used to increase the calcium amount in the body; also it is used as an antacid to treat acidity. It is a constituent compound that makes up the shell of an egg of animals.
Complete answer:
When a compound is formed by the transfer of electrons from one atom to another, then it is termed as an ionic compound. Ionic compounds consist of ions cation and anion. Cations have a positive charge as they donate their valence electron to fulfill their octet, and anions gain electrons to complete their octet. The structural formula of ionic compounds is written in their respective ionic symbols as they are not bonded by electrons, but formed through electron transfer.
We have been given to give the formula for $CaC{{O}_{3}}$ which is an ionic compound. It contains calcium as a cation and carbonate ion as an anion. Calcium loses 1 electron from its valence shell to become $C{{a}^{+}}$ this electron is taken up by one carbonate anion to complete their octet and forms $C{{O}_{3}}^{-}$. The structural formulas of them are:
Calcium is present as $C{{a}^{+}}$ ,
While carbonate as

Calcium is in a form of cation as it is a monatomic ion, while carbonate being a polyatomic ion has a trigonal planar shape.
Hence the structural formula of $CaC{{O}_{3}}$ is

Note:
Calcium carbonate is an important ionic compound. It is formed from the alkali metal calcium which is a constituent part of bones. It is therefore used to increase the calcium amount in the body; also it is used as an antacid to treat acidity. It is a constituent compound that makes up the shell of an egg of animals.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




