
Strong oxidizing agent used in purification of water.
A. $C{{l}_{2}}O$
B. $N{{O}_{3}}^{-}$
C. $N{{O}_{2}}^{-}$
D. $O{{F}_{2}}$
Answer
578.7k+ views
Hint: Oxidizing agent is strong if it can reduce itself to a high amount. Only then can the other atom present in the electrolyte be able to oxidize itself. For that to happen, the oxidizing agent is chosen such that the anion formed after its reduction is stable so as to not react with the compounds again.
Complete step by step solution:
-Based on the property of the compounds being able to dissociate into their ions, they are classified in 2 groups- electrolytes and non-electrolytes. Electrolytes are further classified as strong and weak electrolytes.
-Electrolyte is a compound which dissociates into its constituent ions, cation and anion in presence of DC current under the process of electrolysis.
-Many types of compound can be used as electrolytes but the most preferred compounds are acids, bases and salts. The electrolytes give redox reactions for dissociation into their ions.
-Electrolysis is a technique used to separate a compound in its different constituents with the help of DC current. It is used in non-spontaneous chemical reactions.
-The amount of voltage to be given depends on the type of the electrolyte used. The voltage is called decomposition potential.
-There are two electrodes, cathode and anode dipped in the electrolyte. They are connected to the battery. In acidified water, water is mixed with dilute sulfuric acid. This mixture acts as electrolyte.
-Cathode is the electrode where the cations are attracted. Reduction takes place at cathode. Anode is the electrode where the anions are attracted. Oxidation takes place at anode.
-There are two types of agents. One is the oxidizing agents and the other is the reducing agents. Oxidizing agents are those which oxidize the other atom by reducing themselves. Reducing agents are those that reduce the other atom by oxidizing themselves.
-Oxidizing agent is strong if it is present in its maximum oxidation state and can be reduced to form a stable ion such that the other species gets oxidized. Water also needs a strong oxidizing agent so that the oxygen atom oxidizes from -2 state to 0 state.
-In the given options, the oxidation state of the following central atoms are +1,+5,+3 and +2 respectively. So except for chlorine, all other atoms cannot be reduced easily so as to oxidize water.
-Chlorine has vacant d-orbitals which is absent in $O$ and $N$ and so it can show variable covalencies as
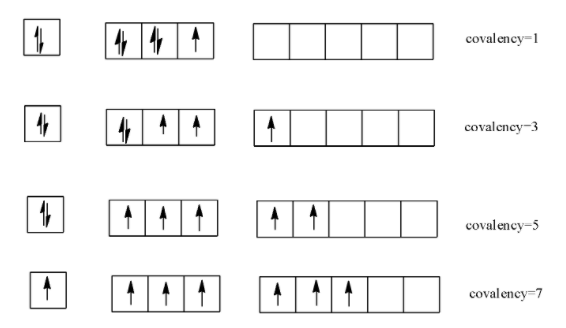
-Thus we see that chlorine can have different oxidation states and this makes it capable to form different compounds with different products. It reduces itself easily and oxidizes water and so it acts as a strong oxidizing agent.
Therefore the correct option is A.
Note: Ozone and hydrogen peroxide are also used for the purification of water as they can also reduce themselves easily from -1 to -2. $C{{l}_{2}}O$ is reduced from +1 to -1 during the process and so is a good oxidizing agent and purifies the water by forming compounds with the impurity molecules.
Complete step by step solution:
-Based on the property of the compounds being able to dissociate into their ions, they are classified in 2 groups- electrolytes and non-electrolytes. Electrolytes are further classified as strong and weak electrolytes.
-Electrolyte is a compound which dissociates into its constituent ions, cation and anion in presence of DC current under the process of electrolysis.
-Many types of compound can be used as electrolytes but the most preferred compounds are acids, bases and salts. The electrolytes give redox reactions for dissociation into their ions.
-Electrolysis is a technique used to separate a compound in its different constituents with the help of DC current. It is used in non-spontaneous chemical reactions.
-The amount of voltage to be given depends on the type of the electrolyte used. The voltage is called decomposition potential.
-There are two electrodes, cathode and anode dipped in the electrolyte. They are connected to the battery. In acidified water, water is mixed with dilute sulfuric acid. This mixture acts as electrolyte.
-Cathode is the electrode where the cations are attracted. Reduction takes place at cathode. Anode is the electrode where the anions are attracted. Oxidation takes place at anode.
-There are two types of agents. One is the oxidizing agents and the other is the reducing agents. Oxidizing agents are those which oxidize the other atom by reducing themselves. Reducing agents are those that reduce the other atom by oxidizing themselves.
-Oxidizing agent is strong if it is present in its maximum oxidation state and can be reduced to form a stable ion such that the other species gets oxidized. Water also needs a strong oxidizing agent so that the oxygen atom oxidizes from -2 state to 0 state.
-In the given options, the oxidation state of the following central atoms are +1,+5,+3 and +2 respectively. So except for chlorine, all other atoms cannot be reduced easily so as to oxidize water.
-Chlorine has vacant d-orbitals which is absent in $O$ and $N$ and so it can show variable covalencies as

-Thus we see that chlorine can have different oxidation states and this makes it capable to form different compounds with different products. It reduces itself easily and oxidizes water and so it acts as a strong oxidizing agent.
Therefore the correct option is A.
Note: Ozone and hydrogen peroxide are also used for the purification of water as they can also reduce themselves easily from -1 to -2. $C{{l}_{2}}O$ is reduced from +1 to -1 during the process and so is a good oxidizing agent and purifies the water by forming compounds with the impurity molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




