
How many stereoisomers are possible for 3, 4-dimethylheptane? Which are pairs of enantiomers and which are meso compounds?
Answer
538.8k+ views
Hint: Stereoisomerism is a kind of isomerism where structure differs in spatial arrangement of atoms rather than atom to atom connectivity. Attempt this question by finding the chiral centers followed by making different arrangement substituents around those chiral centers and then classify them as enantiomers and meso compounds.
Complete answer:
Let us first begin with the basic definitions of chiral centers, enantiomers and meso compounds.
-Chiral center: It is a centre where a tetrahedral atom has 4 different substituent (atom, group or ion) attached to it. Chiral centers of carbon atoms are also known as asymmetric carbon atoms.
-Enantiomers: These are chiral molecules which are non – superimposable mirror images of each other (i.e., they cannot overlap each other just like our hands).
-Meso compounds: These are the optically inactive molecules even after having multiple chiral centers. To check meso compounds, we must look for an internal plane so that both parts on each side of the plane cancel each other out, giving us an achiral compound.
Now we will draw the structure of 3, 4-dimethylheptane and find the chiral centers:-
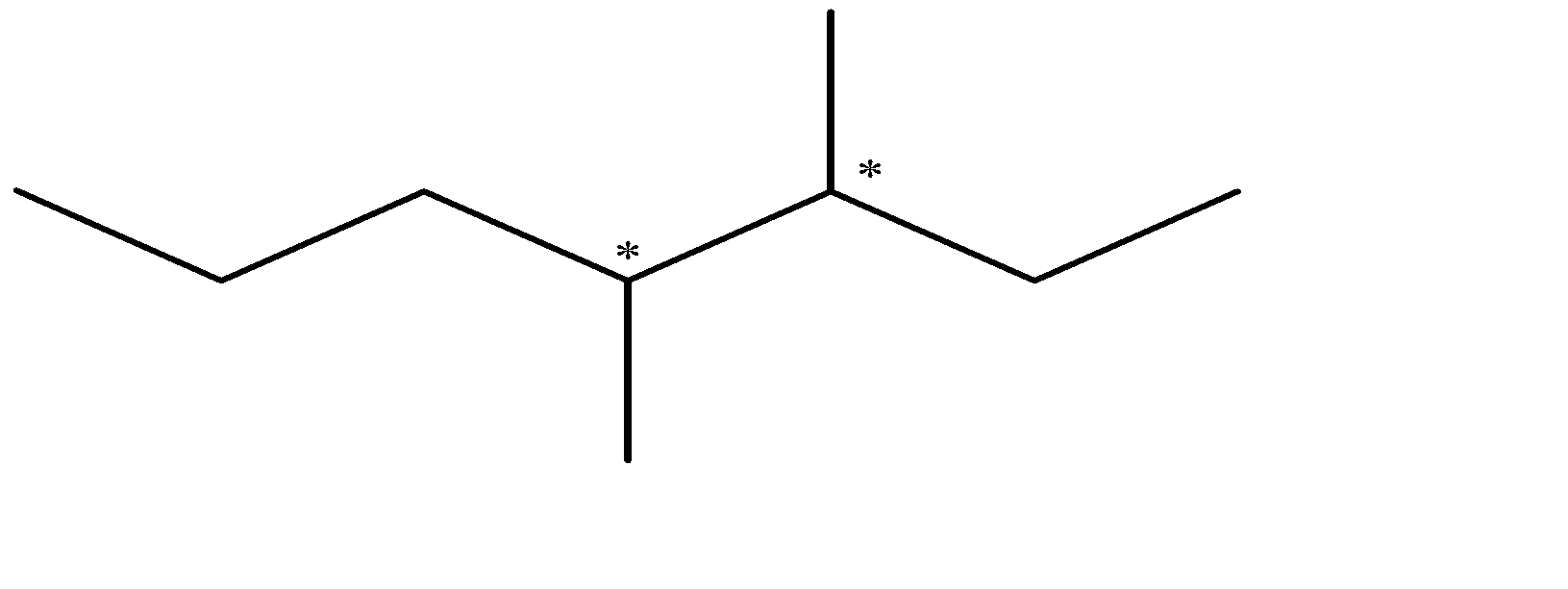
As we can see that there are two chiral centers, therefore we will keep changing the spatial arrangement around them till we get our all possible isomers (all unique).
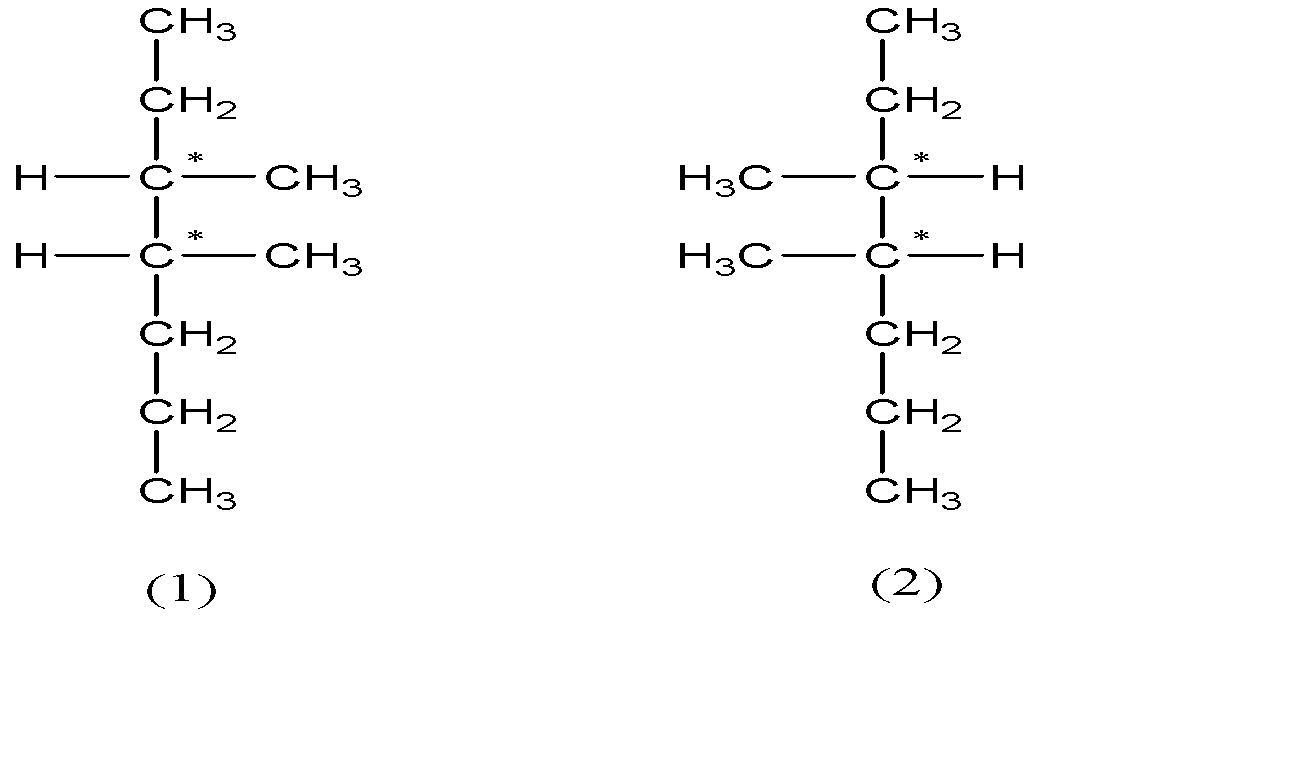
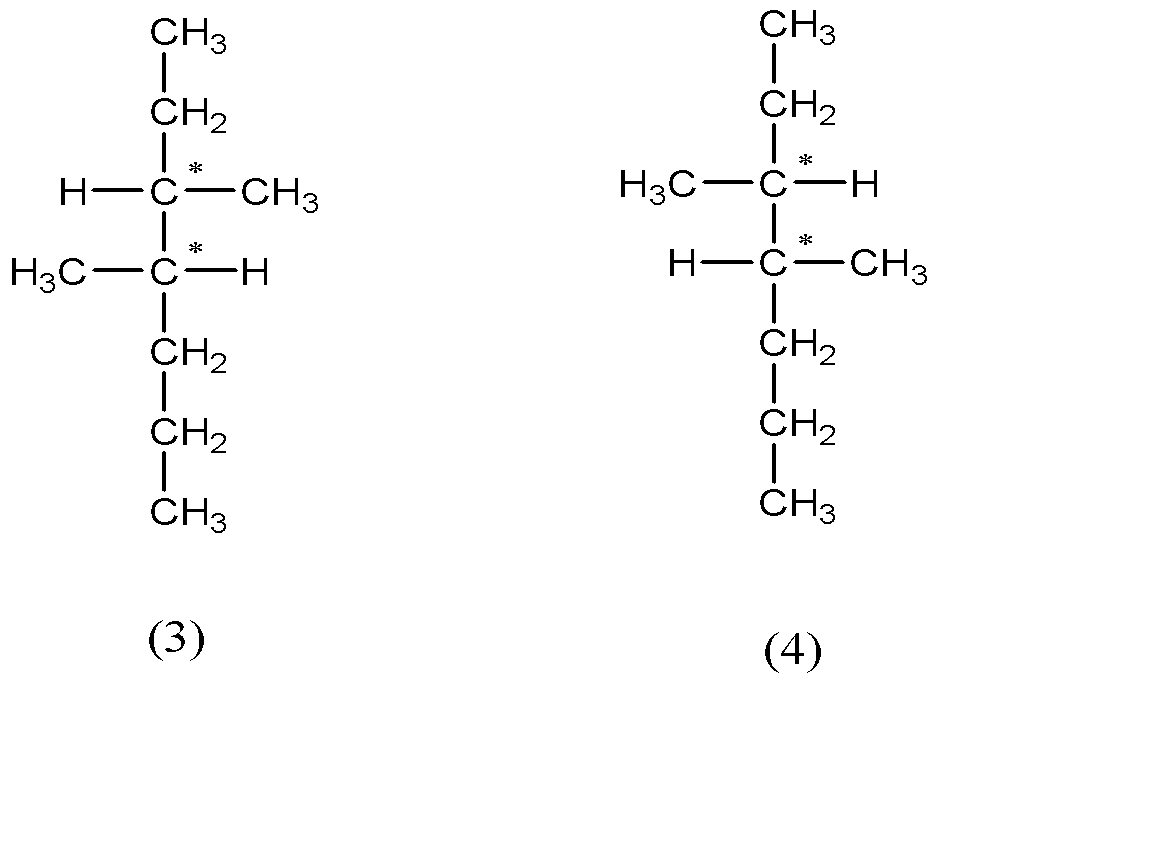
So, there are 4 stereoisomers possible for 3, 4-dimethylheptane.
-From the above structures we can see that (1) and (2) are non - superimposable mirror images of each other and the same goes for (3) and (4). Therefore (1) and (2) ; (3) and (4) are the 2 enantiomeric pairs. Also there is no internal plane in these compounds.
Hence the number of pairs of enantiomers = 2 and there are no meso compounds.
Note:
If there is ‘n’ no. of chiral centre present in a huge unsymmetrical compound, then you can use the formula:-
Total no. of stereoisomer of a molecule or compound = ${{2}^{n}}$
As in case of 3, 4-dimethylheptane, there are 2 chiral centers. Therefore number of stereoisomers = ${{2}^{2}}=4$
-On the basis of chiral centers, we cannot call a molecule to be actively active. This can be explained by the example of meso compounds as they have multiple chiral centers but overall optical activity is null.
Complete answer:
Let us first begin with the basic definitions of chiral centers, enantiomers and meso compounds.
-Chiral center: It is a centre where a tetrahedral atom has 4 different substituent (atom, group or ion) attached to it. Chiral centers of carbon atoms are also known as asymmetric carbon atoms.
-Enantiomers: These are chiral molecules which are non – superimposable mirror images of each other (i.e., they cannot overlap each other just like our hands).
-Meso compounds: These are the optically inactive molecules even after having multiple chiral centers. To check meso compounds, we must look for an internal plane so that both parts on each side of the plane cancel each other out, giving us an achiral compound.
Now we will draw the structure of 3, 4-dimethylheptane and find the chiral centers:-

As we can see that there are two chiral centers, therefore we will keep changing the spatial arrangement around them till we get our all possible isomers (all unique).


So, there are 4 stereoisomers possible for 3, 4-dimethylheptane.
-From the above structures we can see that (1) and (2) are non - superimposable mirror images of each other and the same goes for (3) and (4). Therefore (1) and (2) ; (3) and (4) are the 2 enantiomeric pairs. Also there is no internal plane in these compounds.
Hence the number of pairs of enantiomers = 2 and there are no meso compounds.
Note:
If there is ‘n’ no. of chiral centre present in a huge unsymmetrical compound, then you can use the formula:-
Total no. of stereoisomer of a molecule or compound = ${{2}^{n}}$
As in case of 3, 4-dimethylheptane, there are 2 chiral centers. Therefore number of stereoisomers = ${{2}^{2}}=4$
-On the basis of chiral centers, we cannot call a molecule to be actively active. This can be explained by the example of meso compounds as they have multiple chiral centers but overall optical activity is null.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




