
Statement 1: $NO_3^-$ and $CO_3^{2-}$ are planar species.
Statement 2: Each ion has three equal bonds distributed equivalently in space.
(A) Both Statement $1$ and Statement $2$ are correct and Statement $2$ is the correct explanation of Statement $1$.
(B) Both Statement $1$ and Statement $2$ are correct, but Statement $2$ is NOT the correct explanation of Statement $1$.
(C) Statement $1$ is correct, but Statement $2$ is not correct.
(D) Statement $1$ is not correct, but Statement $2$ is correct.
Answer
564.6k+ views
Hint: The shape of a molecule depends upon the hybridization of the molecule, and the repulsion between the bonding atoms. Hybridization is defined as the combination of atomic orbitals of similar energy to form new orbitals. A molecule will have a planar shape if it has a hybridization of $sp^2$.
Complete step by step answer:
The $NO_3^-$ ion consists of nitrogen as the central atom, surrounded by three oxygen atoms as shown in the following diagram:
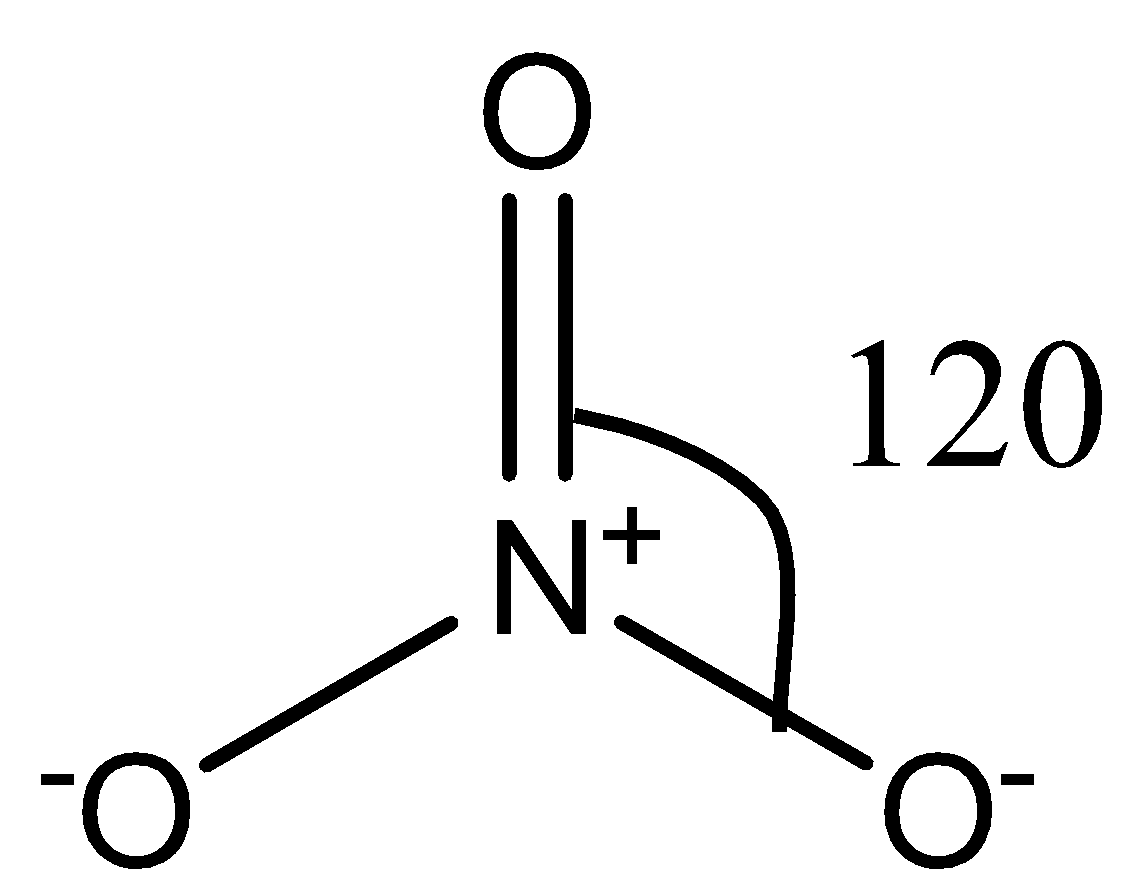
There are no lone pairs on the nitrogen atom. So, there is no lone pair-bond pair repulsion in the molecule. There are three sigma bonds in the molecule, so the hybridization in the molecule is $sp^2$.
Now, there will be repulsion between the oxygen atoms that are surrounding the central nitrogen atom. Since the surrounding atoms are all identical, i.e., oxygen, the repulsion between the oxygen atoms will also be the same. Due to this repulsion, the oxygen atoms align themselves at an angle of $120\circ$ to each other. Therefore, the ion has a trigonal planar shape.
Like $NO_3^-$ ion, $CO_3^{2-}$ ion also consists of three oxygen atoms surrounding a central carbon atom. The structure of the $CO_3^{2-}$ ion can be represented through the following diagram:
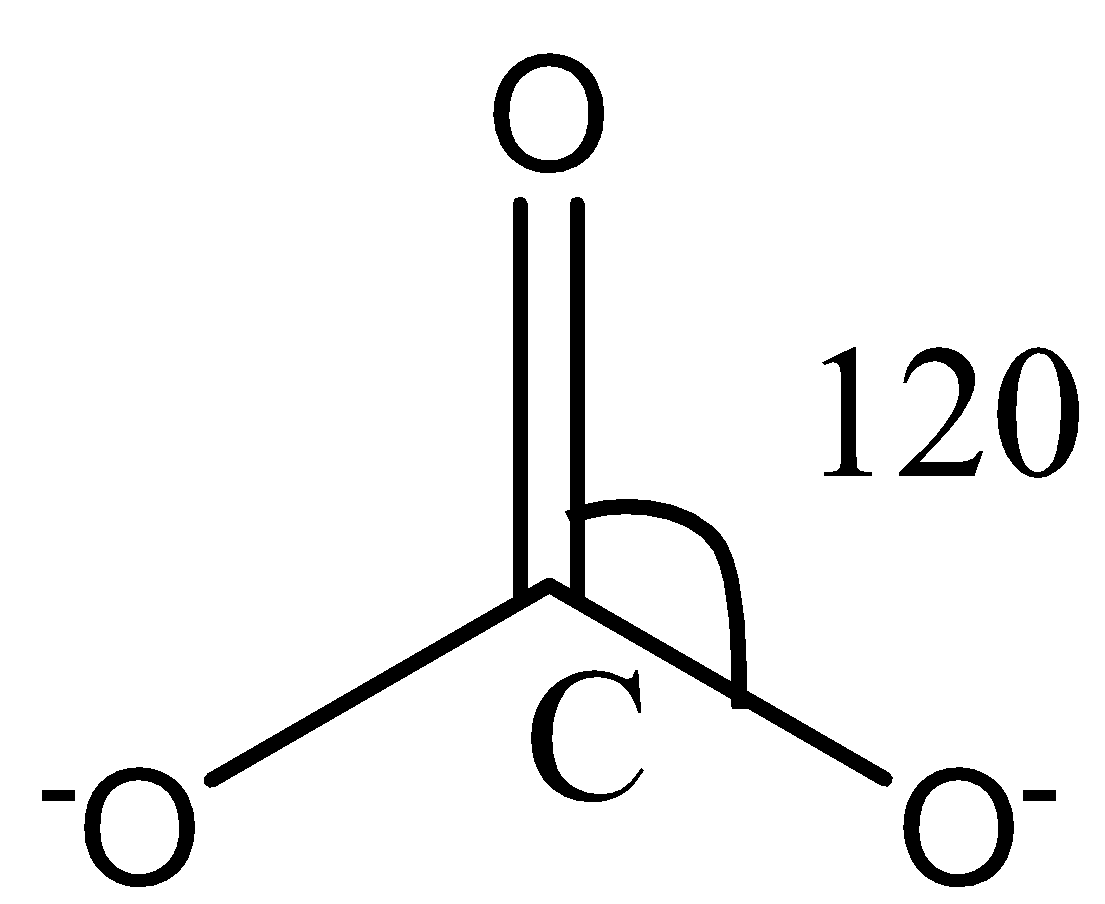
Again, in the carbonate ion, there are no lone pairs on the carbon atom, and therefore, no lone pair-bond pair repulsion in the molecule. The hybridization in the molecule is also $sp_2$ as there are three sigma bonds.
Also, the surrounding atoms are the same as that in the nitrate ion. The three oxygen atoms will repel each other and form an angle of $120\circ$ with each other to attain stability.
Therefore, in the case of both $CO_3^{2-}$ and $NO_3^-$, the bond angle is $120\circ$. Therefore, they both are trigonal planar species, and it is also true that both the ions have three equal bonds distributed equivalently in space at angles of $120 ^\circ$ each. But it is not true that because of an equivalent distribution in space, the molecules have a planar shape. The planar shape of the molecule is because the hybridization of both the molecules is $sp^2$, and there is no lone pair-bond pair repulsion in the molecules.
Therefore, both statements $1$ and $2$ are correct, but statement $2$ is not the correct explanation for statement $1$.
So, the correct answer is Option B.
Note: Hybridization always involves only one $s-$ orbital and the corresponding number of $p-$ orbitals as per the number of sigma bonds. The sigma bonds in a molecule are the single bonds in the molecule. In the case of both $NO_3^-$ and $CO_3^{2-}$, the number of sigma or single bonds is $3$. So, the total number of orbitals including $s-$ and $p-$ should also be three. Therefore, the hybridization of both the given ions can be simply deduced to be $Sp^2$ (one $s-$ orbital and two $p-$ orbitals).
Complete step by step answer:
The $NO_3^-$ ion consists of nitrogen as the central atom, surrounded by three oxygen atoms as shown in the following diagram:

There are no lone pairs on the nitrogen atom. So, there is no lone pair-bond pair repulsion in the molecule. There are three sigma bonds in the molecule, so the hybridization in the molecule is $sp^2$.
Now, there will be repulsion between the oxygen atoms that are surrounding the central nitrogen atom. Since the surrounding atoms are all identical, i.e., oxygen, the repulsion between the oxygen atoms will also be the same. Due to this repulsion, the oxygen atoms align themselves at an angle of $120\circ$ to each other. Therefore, the ion has a trigonal planar shape.
Like $NO_3^-$ ion, $CO_3^{2-}$ ion also consists of three oxygen atoms surrounding a central carbon atom. The structure of the $CO_3^{2-}$ ion can be represented through the following diagram:

Again, in the carbonate ion, there are no lone pairs on the carbon atom, and therefore, no lone pair-bond pair repulsion in the molecule. The hybridization in the molecule is also $sp_2$ as there are three sigma bonds.
Also, the surrounding atoms are the same as that in the nitrate ion. The three oxygen atoms will repel each other and form an angle of $120\circ$ with each other to attain stability.
Therefore, in the case of both $CO_3^{2-}$ and $NO_3^-$, the bond angle is $120\circ$. Therefore, they both are trigonal planar species, and it is also true that both the ions have three equal bonds distributed equivalently in space at angles of $120 ^\circ$ each. But it is not true that because of an equivalent distribution in space, the molecules have a planar shape. The planar shape of the molecule is because the hybridization of both the molecules is $sp^2$, and there is no lone pair-bond pair repulsion in the molecules.
Therefore, both statements $1$ and $2$ are correct, but statement $2$ is not the correct explanation for statement $1$.
So, the correct answer is Option B.
Note: Hybridization always involves only one $s-$ orbital and the corresponding number of $p-$ orbitals as per the number of sigma bonds. The sigma bonds in a molecule are the single bonds in the molecule. In the case of both $NO_3^-$ and $CO_3^{2-}$, the number of sigma or single bonds is $3$. So, the total number of orbitals including $s-$ and $p-$ should also be three. Therefore, the hybridization of both the given ions can be simply deduced to be $Sp^2$ (one $s-$ orbital and two $p-$ orbitals).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




