
State the meaning of functional group in a carbon compound. Write the functional group present in
(a) ethanol
(b) ethanoic acid
And draw their structures.
Answer
566.7k+ views
Hint: In organic chemistry, functional groups are specific substituents or moieties within molecules that may be responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of.
Complete step by step answer:
We have been asked about the functional group,
So, for that:
- Functional groups are specific groupings of atoms within molecules that have their own characteristic properties, regardless of the other atoms present in a molecule. Common examples are alcohols, amines, carboxylic acids, ketones, and ethers.
We have been given ethanol whose structure is:
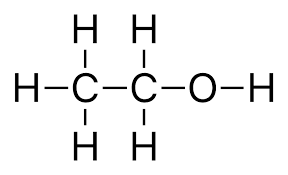
In this the functional group is hydroxyl-OH,
So, it belongs to the alcohol group.
Then we have, ethanoic acid whose structure is:
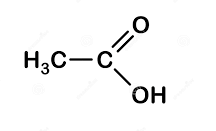
- In this the functional group is carboxyl-COOH,
So, it belongs to the carboxylic acid group.
So, we can say that the functional group present in ethanol is alcohol and ethanoic acid is carboxylic acid.
Note: CARBOXYLIC ACIDS (highest priority among carbon-containing functional groups).
-CARBOXYLIC ACID DERIVATIVES.
-OTHER GROUPS CONTAINING OXYGEN OR NITROGEN.
-ALKENES AND ALKYNES.
Note: substances containing double and triple bonds are called alkenynes. (notice that the name ends in yne).
Complete step by step answer:
We have been asked about the functional group,
So, for that:
- Functional groups are specific groupings of atoms within molecules that have their own characteristic properties, regardless of the other atoms present in a molecule. Common examples are alcohols, amines, carboxylic acids, ketones, and ethers.
| Organic compound | Functional group |
| Haloalkanes | Halide-X(Cl, F, Br, I) |
| Alcohols | Hydroxyl-OH |
| Aldehydes | Hydroxyl-CHO |
| Carboxylic acid | Carboxyl-COOH |
| Ketones | Keto, 
|
| Ethers | Ethers
|
We have been given ethanol whose structure is:

In this the functional group is hydroxyl-OH,
So, it belongs to the alcohol group.
Then we have, ethanoic acid whose structure is:

- In this the functional group is carboxyl-COOH,
So, it belongs to the carboxylic acid group.
So, we can say that the functional group present in ethanol is alcohol and ethanoic acid is carboxylic acid.
Note: CARBOXYLIC ACIDS (highest priority among carbon-containing functional groups).
-CARBOXYLIC ACID DERIVATIVES.
-OTHER GROUPS CONTAINING OXYGEN OR NITROGEN.
-ALKENES AND ALKYNES.
Note: substances containing double and triple bonds are called alkenynes. (notice that the name ends in yne).
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




