
State the law of conservation of mass. Give one example to illustrate this law.
Answer
566.1k+ views
Hint: In a balanced chemical equation, the number of atoms taking part in the reaction to form a molecule is the same on both sides of the reaction. Ultimately it provides the conclusion that the total mass involved in any reaction remains the same.
Step by step answer: The given question is very straightforward as it asks you to state the law of conservation of mass, with the appropriate example. The example can be given on the basis of the daily life application of the law. Here, one needs to read the question properly as the word mass change- in the law of conservation of mass we can write law of conservation of matter-by matter the example changes, but all other words in the law remain the same.
The law of conservation of mass is derived from the chemical changes that are seen in our daily life, the law is derived and it is based on n-number of observations made by the scientists. Whenever there is change in the state of the molecule after reaction or breaking or formation of the bond the mass of the product form is equal to the mass of the reactants. The properties of the reactants are not similar to that of the product formed by the amount. It is like a production machine: what you put in you will get out of the product after the appropriate physical or chemical changes that are done as per the set procedure.
Law of conservation of mass says that “mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants.”
Whenever the chemical change takes place there is change in the reactant chemically to form products that have different properties by the amount of reactant will always remain the same as that of the product.
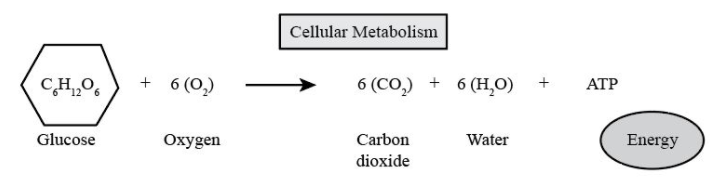
Example reactants like glucose in presence of oxygen will give us products like carbon dioxide and water with energy due to breakage of bond during cellular respiration. Reaction is as shown, the number of molecules are the same on reactants and product sides.
Note: As we know the law of conservation of mass is similar to that of the law of conservation of matter, therefore we should not get confused between them. It's a natural law which states that anyhow represents that mass neither can be created nor destroyed.
Step by step answer: The given question is very straightforward as it asks you to state the law of conservation of mass, with the appropriate example. The example can be given on the basis of the daily life application of the law. Here, one needs to read the question properly as the word mass change- in the law of conservation of mass we can write law of conservation of matter-by matter the example changes, but all other words in the law remain the same.
The law of conservation of mass is derived from the chemical changes that are seen in our daily life, the law is derived and it is based on n-number of observations made by the scientists. Whenever there is change in the state of the molecule after reaction or breaking or formation of the bond the mass of the product form is equal to the mass of the reactants. The properties of the reactants are not similar to that of the product formed by the amount. It is like a production machine: what you put in you will get out of the product after the appropriate physical or chemical changes that are done as per the set procedure.
Law of conservation of mass says that “mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants.”
Whenever the chemical change takes place there is change in the reactant chemically to form products that have different properties by the amount of reactant will always remain the same as that of the product.
Example reactants like glucose in presence of oxygen will give us products like carbon dioxide and water with energy due to breakage of bond during cellular respiration. Reaction is as shown, the number of molecules are the same on reactants and product sides.

Note: As we know the law of conservation of mass is similar to that of the law of conservation of matter, therefore we should not get confused between them. It's a natural law which states that anyhow represents that mass neither can be created nor destroyed.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




