
State the electronic configuration for aluminium [p=13, n=14]
[A] 2, 8, 2
[B] 2, 8, 3
[C] 2, 8, 4
[D] 2, 8, 5
Answer
584.4k+ views
HINT: The atomic number of aluminium is 13. We can follow the octet rule to write the configuration according to K-shell can accommodate 2 electrons L-shell can accommodate 8 electrons and the remaining 3 electrons will be in the M-shell.
COMPLETE STEP BY STEP SOLUTION:
Electronic configuration of any atom or molecule gives us the numeric arrangement of electrons around the nucleus.
There are specific notations that we use for writing the configuration of any atom. For writing these notations, we start with the energy orbitals. Practically, there are 4 orbitals s, p, d and f. There is a certain even number of electrons that each orbital can accommodate. s-orbital can accommodate 2 electrons whereas p, d and f-orbitals can accommodate 6, 8 and 14 electrons respectively.
There is a trend each electron follows while filling these orbitals and it is given as-
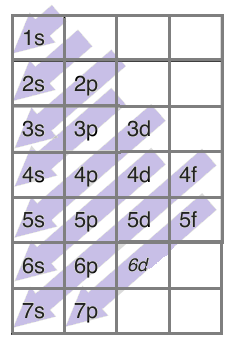
For example, if there are 6 electrons in an element, two electrons will enter 1s orbital first followed by two electrons in 2s-orbital and then the remaining 2 in the 2p-orbital.
Similarly, we can write the electronic configuration for aluminium.
The atomic number of aluminium is 13 which means it has 13 electrons and 13 protons. We can write the electronic configuration for 13 electrons following the above diagram as-$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{1}}$.
If we consider the total number of electrons in each energy level i.e. n= 1, 2 and 3, we can write that the total number of electrons in K-shell is 2$\left( 1{{s}^{2}} \right)$, in L-shell is 8$\left( 2{{s}^{2}}2{{p}^{6}} \right)$ and M-shell is 3$\left( 3{{s}^{2}}3{{p}^{1}} \right)$
Therefore, we can write the electronic configuration of aluminum as 2, 8, 3.
Therefore, the correct answer is option [B] 2, 8, 3.
NOTE: According to Pauli’s Exclusion Principle, each orbital can hold 2 electrons. s-orbital set contains one orbital, thus can hold 2 electrons. Similarly, the p-orbital set had three orbitals, thus can hold 6 electrons and d-orbital and f-orbital have five and seven orbitals thus, can hole 10 and 14 electrons respectively.
COMPLETE STEP BY STEP SOLUTION:
Electronic configuration of any atom or molecule gives us the numeric arrangement of electrons around the nucleus.
There are specific notations that we use for writing the configuration of any atom. For writing these notations, we start with the energy orbitals. Practically, there are 4 orbitals s, p, d and f. There is a certain even number of electrons that each orbital can accommodate. s-orbital can accommodate 2 electrons whereas p, d and f-orbitals can accommodate 6, 8 and 14 electrons respectively.
There is a trend each electron follows while filling these orbitals and it is given as-

For example, if there are 6 electrons in an element, two electrons will enter 1s orbital first followed by two electrons in 2s-orbital and then the remaining 2 in the 2p-orbital.
Similarly, we can write the electronic configuration for aluminium.
The atomic number of aluminium is 13 which means it has 13 electrons and 13 protons. We can write the electronic configuration for 13 electrons following the above diagram as-$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{1}}$.
If we consider the total number of electrons in each energy level i.e. n= 1, 2 and 3, we can write that the total number of electrons in K-shell is 2$\left( 1{{s}^{2}} \right)$, in L-shell is 8$\left( 2{{s}^{2}}2{{p}^{6}} \right)$ and M-shell is 3$\left( 3{{s}^{2}}3{{p}^{1}} \right)$
Therefore, we can write the electronic configuration of aluminum as 2, 8, 3.
Therefore, the correct answer is option [B] 2, 8, 3.
NOTE: According to Pauli’s Exclusion Principle, each orbital can hold 2 electrons. s-orbital set contains one orbital, thus can hold 2 electrons. Similarly, the p-orbital set had three orbitals, thus can hold 6 electrons and d-orbital and f-orbital have five and seven orbitals thus, can hole 10 and 14 electrons respectively.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




