
What starting material is needed to synthesize the following compounds?





Answer
512.4k+ views
Hint: Alkenes are unsaturated compounds consisting of double bonds and when alkenes treated with potassium permanganate, diols were formed. The potassium permanganate is an oxidizing agent. The diols formed when treated with sodium hydride, a base then the above products will be formed.
Complete answer:
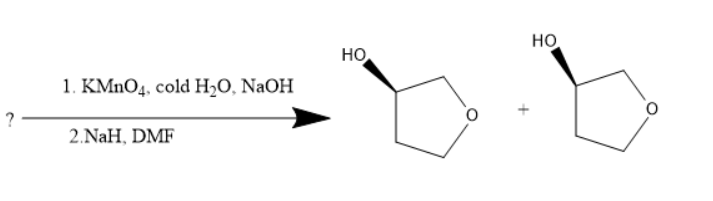
Alkenes are unsaturated compounds due to the presence of double bonds. Alkenes when treated with potassium permanganate undergoes an additional reaction. The diols will be formed. Diol is a compound with two hydroxyl groups in the molecule. When this diol is treated with sodium hydride and dimethyl formamide, cyclisation occurs and oxygen atom goes into the ring and thus the compound formed has the hydroxyl substituent and cyclo butoxy compound.
Potassium permanganate is an oxidizing agent and sodium hydroxide is a strong base. Both these compounds are used for the diol’s formation. The sodium hydride belongs to the hydride family, a strong base and dimethylformamide can be used as a solvent.
The reaction will be as follows:
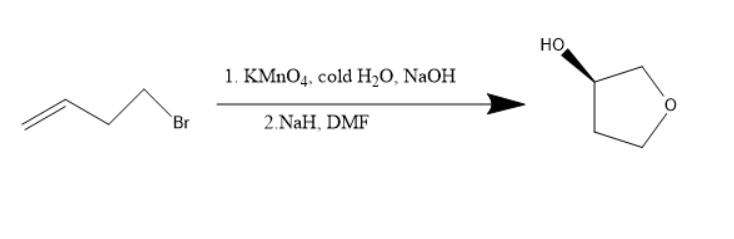
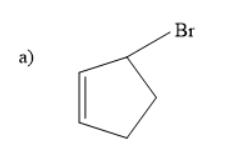
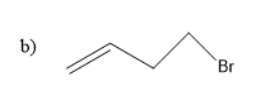
Thus, the starting material is an alkene with bromine as substituent.
Thus, \[1\]-bromo but- \[4\]-ene is the starting material.
So, the correct answer is “Option b”.
Note:
Alkenes are also known as olefins. Due to the presence of double bond, alkenes undergo addition reactions; the addition of potassium permanganate in presence of a base is an addition reaction to form diols. The diols further treatment with sodium hydride forms a cyclo alkoxy compound.
Complete answer:
Alkenes are unsaturated compounds due to the presence of double bonds. Alkenes when treated with potassium permanganate undergoes an additional reaction. The diols will be formed. Diol is a compound with two hydroxyl groups in the molecule. When this diol is treated with sodium hydride and dimethyl formamide, cyclisation occurs and oxygen atom goes into the ring and thus the compound formed has the hydroxyl substituent and cyclo butoxy compound.
Potassium permanganate is an oxidizing agent and sodium hydroxide is a strong base. Both these compounds are used for the diol’s formation. The sodium hydride belongs to the hydride family, a strong base and dimethylformamide can be used as a solvent.
The reaction will be as follows:

Thus, the starting material is an alkene with bromine as substituent.
Thus, \[1\]-bromo but- \[4\]-ene is the starting material.
So, the correct answer is “Option b”.
Note:
Alkenes are also known as olefins. Due to the presence of double bond, alkenes undergo addition reactions; the addition of potassium permanganate in presence of a base is an addition reaction to form diols. The diols further treatment with sodium hydride forms a cyclo alkoxy compound.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




