
Why do some people call $ {({H_3}C)_4}C $ as neopentane instead of dimethyl propane?
Answer
493.5k+ views
Hint: There are two types of names that are given to chemical compounds, especially organic compounds. These names are IUPAC names and common names. The IUPAC names are given according to the rules set for the nomenclature of organic compounds by the IUPAC (International Union of Pure and Applied Chemistry) while there are no strict rules to give the common names to the compounds.
Complete Step By Step Answer:
We should know that there are two types of names that are given to chemical compounds, especially organic compounds. One name is common name and the other name is IUPAC name. The IUPAC names are given according to the rules set for the nomenclature of organic compounds by the IUPAC (International Union of Pure and Applied Chemistry) while there are no strict rules to give the common names to the compounds.
First we will see the structure of dimethylpropane:
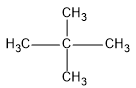
So, as the name suggests, there is a propane chain (longest carbon chain) and two methyl groups are present at the second position of the propane chain.
This compound is also known as neopentane because neopentane is the common name for this compound. This common name is given because dimethylpropane is an isomer of pentane.
Note:
We should know that neopentane or dimethyl propane is a double branched alkane. It contains five carbon atoms in its structure. At room temperature and pressure, it is a flammable gas which can be condensed into a volatile liquid when the temperature is lower down (in winters or when kept in an ice bath).
Complete Step By Step Answer:
We should know that there are two types of names that are given to chemical compounds, especially organic compounds. One name is common name and the other name is IUPAC name. The IUPAC names are given according to the rules set for the nomenclature of organic compounds by the IUPAC (International Union of Pure and Applied Chemistry) while there are no strict rules to give the common names to the compounds.
First we will see the structure of dimethylpropane:

So, as the name suggests, there is a propane chain (longest carbon chain) and two methyl groups are present at the second position of the propane chain.
This compound is also known as neopentane because neopentane is the common name for this compound. This common name is given because dimethylpropane is an isomer of pentane.
Note:
We should know that neopentane or dimethyl propane is a double branched alkane. It contains five carbon atoms in its structure. At room temperature and pressure, it is a flammable gas which can be condensed into a volatile liquid when the temperature is lower down (in winters or when kept in an ice bath).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




