
Why do $ SN2 $ reactions prefer aprotic solvents?
Answer
503.1k+ views
Hint: Aprotic solvents are neutral solvents. They increase the rate of $ SN2 $ reaction. The $ SN2 $ reaction is a bimolecular nucleophilic substitution reaction. The rate of the reaction depends on the concentration of both the nucleophile and the molecule undergoing attack.
Complete answer:
The $ SN2 $ reaction is a nucleophilic substitution reaction where a bond is broken and another is formed simultaneously. The term ‘ $ SN2 $ ’ means Substitution nucleophilic bimolecular and so this type of reaction is also referred to as bimolecular nucleophilic substitution reaction. In this reaction, the rate of the reaction is determined by two reacting species.
During the mechanism of $ SN2 $ reactions, some of the factors are considered, such as the presence of a strong anionic nucleophile, stability of the anion, and presence of aprotic solvents. The SN2 reactions usually prefer aprotic solvents.
The aprotic solvents are neutral and they neither donate nor accept any proton.
A solvent is required in these reactions to facilitate the collision between the nucleophile and the electrophile.
Let us take an example of the aprotic solvent DMSO (Dimethyl sulfoxide):
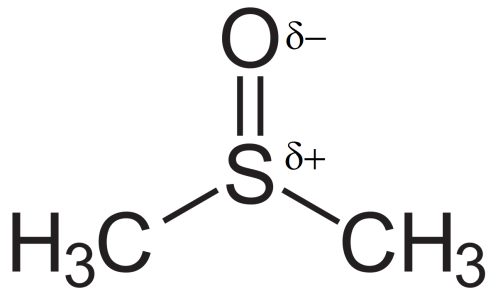
We see here that this solvent has a $ \delta - {\text{ }}and{\text{ }}\delta + $ charge. Due to this $ \delta - $ it becomes easy for the solvent to solvate the counter ion thereby releasing the nucleophile for attack. Also as the $ \delta + $ is crowded it will not be able to solvate the nucleophile. Thus the nucleophile is free to attack the substrate. Whereas if there is a protic solvent present, it will solvate both the counter ion as well as the nucleophile and so the nucleophile is not free to attack.
Note:
The $ SN2 $ reactions are the single step reactions. There is no intermediate formation. This reaction does not produce any carbocation too. This reaction product of this reaction is inversion of the reaction and it is called Walden's inversion.
Complete answer:
The $ SN2 $ reaction is a nucleophilic substitution reaction where a bond is broken and another is formed simultaneously. The term ‘ $ SN2 $ ’ means Substitution nucleophilic bimolecular and so this type of reaction is also referred to as bimolecular nucleophilic substitution reaction. In this reaction, the rate of the reaction is determined by two reacting species.
During the mechanism of $ SN2 $ reactions, some of the factors are considered, such as the presence of a strong anionic nucleophile, stability of the anion, and presence of aprotic solvents. The SN2 reactions usually prefer aprotic solvents.
The aprotic solvents are neutral and they neither donate nor accept any proton.
A solvent is required in these reactions to facilitate the collision between the nucleophile and the electrophile.
Let us take an example of the aprotic solvent DMSO (Dimethyl sulfoxide):

We see here that this solvent has a $ \delta - {\text{ }}and{\text{ }}\delta + $ charge. Due to this $ \delta - $ it becomes easy for the solvent to solvate the counter ion thereby releasing the nucleophile for attack. Also as the $ \delta + $ is crowded it will not be able to solvate the nucleophile. Thus the nucleophile is free to attack the substrate. Whereas if there is a protic solvent present, it will solvate both the counter ion as well as the nucleophile and so the nucleophile is not free to attack.
Note:
The $ SN2 $ reactions are the single step reactions. There is no intermediate formation. This reaction does not produce any carbocation too. This reaction product of this reaction is inversion of the reaction and it is called Walden's inversion.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




