
How many \[\sigma \] and \[\pi \] – bonds are present in peroxymonosulfuric acid?
Answer
592.5k+ views
Hint: So, for finding sigma and pi bonds we have to draw the bond-line structure of peroxymonosulfuric acid and then count each and every bond between any of two atoms or elements.
Complete step by step solution:
Sigma bonds (σ bonds) are the strongest type of covalent chemical bonds. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups.
We know that Pi bond (\[\pi \]bond) is a bond formed by the overlap of p-orbitals on adjacent atoms, perpendicular to any sigma bond(s) between the same atoms. It is indicated in a Kekule structure or bond-line structure as an extra line parallel to the line which represents the sigma bond.
Chemical formula of peroxymonosulfuric acid is: \[{{H}_{2}}S{{O}_{5}}\]
And structure of peroxymonosulfuric acid:
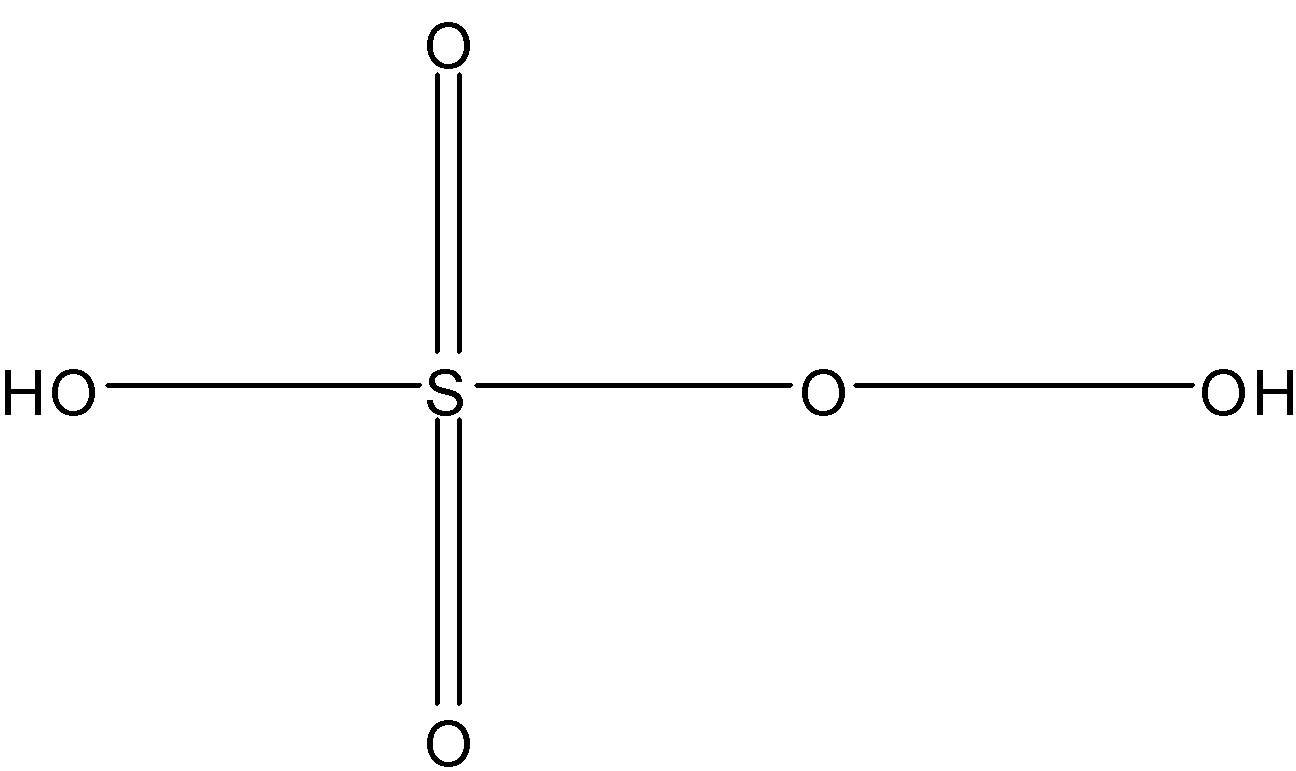
Here, number of sigma bonds = 4 sigma bonds between S and O + 2 sigma bonds between H and O + 1 sigma bond between O and O
= 7
Number of pi bonds = there are two pi bonds between S and O = 2
So, the answer is “7, 2”.
Note: If there is a double or triple bond between any of two atoms then 1st bond we count as sigma bond and other two or one bond we count as pi bond. And in this compound a peroxy bond (O-O).
Complete step by step solution:
Sigma bonds (σ bonds) are the strongest type of covalent chemical bonds. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups.
We know that Pi bond (\[\pi \]bond) is a bond formed by the overlap of p-orbitals on adjacent atoms, perpendicular to any sigma bond(s) between the same atoms. It is indicated in a Kekule structure or bond-line structure as an extra line parallel to the line which represents the sigma bond.
Chemical formula of peroxymonosulfuric acid is: \[{{H}_{2}}S{{O}_{5}}\]
And structure of peroxymonosulfuric acid:

Here, number of sigma bonds = 4 sigma bonds between S and O + 2 sigma bonds between H and O + 1 sigma bond between O and O
= 7
Number of pi bonds = there are two pi bonds between S and O = 2
So, the answer is “7, 2”.
Note: If there is a double or triple bond between any of two atoms then 1st bond we count as sigma bond and other two or one bond we count as pi bond. And in this compound a peroxy bond (O-O).
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




