
How many sigma and pi bonds are in the HCN molecule?
Answer
540.9k+ views
Hint: Hydrogen cyanide is an acid that has molecular formula $H-C\equiv N$. We know that when a pair of electrons are shared by two atoms, a covalent bond is formed. Sigma ($\sigma $) bonds and pi ($\pi $) bonds are two types of covalent bonds.
Complete step by step solution:
The strongest type of covalent chemical bond is a sigma ($\sigma $) bond. A sigma ($\sigma $) bond is formed when the atomic orbitals overlap head-on. Usually, sigma ($\sigma $) bonds are single bonds.
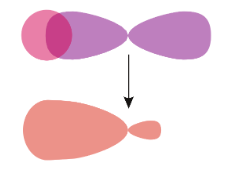
The sigma ($\sigma $) bonds are symmetrical and can rotate about the bond axis. Some of the most common sigma bonds are s+s, ${{p}_{z}}+{{p}_{z}}$, s+${{p}_{z}}$, and ${{d}_{{{z}^{2}}}}+{{d}_{{{z}^{2}}}}$. Here, s, p, and d are atomic orbitals and z is the bond axis.
Now, the covalent chemical bonds which are formed when the atomic orbitals overlap laterally are known as pi ($\pi $) bonds. Usually, there is 1 pi ($\pi $) bond in double bonds and 2 pi ($\pi $) bonds in triple bonds.
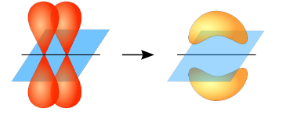
Pi ($\pi $) bonds cannot rotate about the bond axis without breaking the bond.
Now, in HCN, we can see that there are two single bonds, H-C and C-N, hence it has two sigma ($\sigma $) bonds. Whereas it has a triple bond in $C\equiv N$ and hence has two pi ($\pi $) bonds.
So, the HCN molecule has 2 sigma ($\sigma $) bonds and 2 pi ($\pi $) bonds.
Additional Information: Some of the properties of HCN are
- It can be synthesized by combining ammonia and methane
\[2C{{H}_{4}}+2N{{H}_{3}}+3{{O}_{2}}\xrightarrow[\Delta =1200{}^\circ C]{Pt}2HCN+6{{H}_{2}}O\]
- It has an odor of bitter almond oil.
- It exists in both the gaseous as well as the liquid state.
- It has a melting point of 259.86 K and a boiling point of 299.00 K.
- It has a density of 0.69 g/l.
- It has a linear structure.
- It is extremely toxic as well as poisonous in nature and has been used as chemical weapons.
- It is colorless and is soluble and miscible in water and ethanol.
Note: It should be noted that even though pi ($\pi $) bonds are weaker than sigma ($\sigma $) bonds, but when atoms are bonded by both sigma ($\sigma $) bonds and pi ($\pi $) bonds, their strength is greater than either of the bonds alone. Hence the strength of multiple bonds (double and triple bonds) is more than that of a single bond.
Complete step by step solution:
The strongest type of covalent chemical bond is a sigma ($\sigma $) bond. A sigma ($\sigma $) bond is formed when the atomic orbitals overlap head-on. Usually, sigma ($\sigma $) bonds are single bonds.

The sigma ($\sigma $) bonds are symmetrical and can rotate about the bond axis. Some of the most common sigma bonds are s+s, ${{p}_{z}}+{{p}_{z}}$, s+${{p}_{z}}$, and ${{d}_{{{z}^{2}}}}+{{d}_{{{z}^{2}}}}$. Here, s, p, and d are atomic orbitals and z is the bond axis.
Now, the covalent chemical bonds which are formed when the atomic orbitals overlap laterally are known as pi ($\pi $) bonds. Usually, there is 1 pi ($\pi $) bond in double bonds and 2 pi ($\pi $) bonds in triple bonds.

Pi ($\pi $) bonds cannot rotate about the bond axis without breaking the bond.
Now, in HCN, we can see that there are two single bonds, H-C and C-N, hence it has two sigma ($\sigma $) bonds. Whereas it has a triple bond in $C\equiv N$ and hence has two pi ($\pi $) bonds.
So, the HCN molecule has 2 sigma ($\sigma $) bonds and 2 pi ($\pi $) bonds.
Additional Information: Some of the properties of HCN are
- It can be synthesized by combining ammonia and methane
\[2C{{H}_{4}}+2N{{H}_{3}}+3{{O}_{2}}\xrightarrow[\Delta =1200{}^\circ C]{Pt}2HCN+6{{H}_{2}}O\]
- It has an odor of bitter almond oil.
- It exists in both the gaseous as well as the liquid state.
- It has a melting point of 259.86 K and a boiling point of 299.00 K.
- It has a density of 0.69 g/l.
- It has a linear structure.
- It is extremely toxic as well as poisonous in nature and has been used as chemical weapons.
- It is colorless and is soluble and miscible in water and ethanol.
Note: It should be noted that even though pi ($\pi $) bonds are weaker than sigma ($\sigma $) bonds, but when atoms are bonded by both sigma ($\sigma $) bonds and pi ($\pi $) bonds, their strength is greater than either of the bonds alone. Hence the strength of multiple bonds (double and triple bonds) is more than that of a single bond.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




