
Show all the steps to how \[3\]-chloroaniline can synthesize from benzene?
Answer
552.6k+ views
Hint: To solve this question, we need a three-step process to form \[3\]-chloroaniline from benzene. The first step involved is nitration of benzene. The second step is the chlorination of nitrobenzene. The third steps involve the reduction of the nitro group to get our desired product.
Complete step-by-step answer:The first step involves the addition of the nitro group $\left(N O_{2}\right)$ to the benzene ring to form nitrobenzene through nitration of benzene. This can be represented from the diagram below:
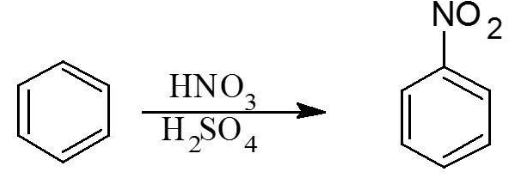
Now, we need to chlorine in the above compound. To do so, we can use the chlorination process since the nitro group is a meta director. The below diagram will make it more clear:
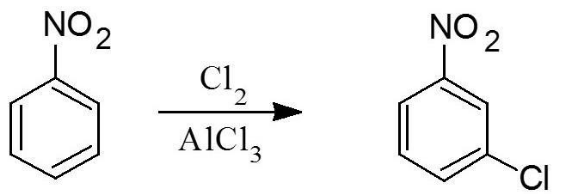
Finally, we have to reduce the \[N{{O}_{2}}\] to form \[N{{H}_{3}}\]. This can be easily done by the process of reduction. The following image will make it more clear:
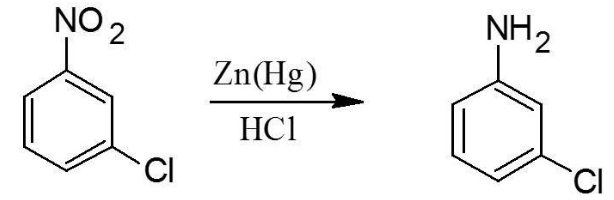
Thus, \[3\]-chloroaniline can be synthesized from benzene in just three easy steps as shown above.
Additional Information:
Benzene has the molecular formula \[{{C}_{6}}{{H}_{6}}\] and is an organic chemical compound. The benzene molecule consists of six atoms of carbon joined in a planar ring with one atom of hydrogen attached to each of them. Benzene is classified as a hydrocarbon as it contains only carbon and hydrogen atoms.
Note:To solve this question or other question which is very similar to this one, it is not necessary to do it the way as shown above. There are many approaches to do the same question. Like alcohol can be synthesised from Grignard reagent and also hydrolysis of esters.
Complete step-by-step answer:The first step involves the addition of the nitro group $\left(N O_{2}\right)$ to the benzene ring to form nitrobenzene through nitration of benzene. This can be represented from the diagram below:

Now, we need to chlorine in the above compound. To do so, we can use the chlorination process since the nitro group is a meta director. The below diagram will make it more clear:

Finally, we have to reduce the \[N{{O}_{2}}\] to form \[N{{H}_{3}}\]. This can be easily done by the process of reduction. The following image will make it more clear:

Thus, \[3\]-chloroaniline can be synthesized from benzene in just three easy steps as shown above.
Additional Information:
Benzene has the molecular formula \[{{C}_{6}}{{H}_{6}}\] and is an organic chemical compound. The benzene molecule consists of six atoms of carbon joined in a planar ring with one atom of hydrogen attached to each of them. Benzene is classified as a hydrocarbon as it contains only carbon and hydrogen atoms.
Note:To solve this question or other question which is very similar to this one, it is not necessary to do it the way as shown above. There are many approaches to do the same question. Like alcohol can be synthesised from Grignard reagent and also hydrolysis of esters.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




