
What is the shape of ethene molecule?
A) Planar
B) Tetrahedral
C) Bipyramidal
D) None of these
Answer
524.1k+ views
Hint: It is easy to identify the shape of a molecule if we know the hybridization of that molecule. Alkanes, Alkenes and Alkynes are the hydrocarbons that contain bonds between carbon and hydrogen atoms.
Complete step by step answer:
We have to know that the ethene has a molecular formula \[{C_2}{H_4}\]. It has a double bond and therefore belongs to alkene.
We can write the general formula of Alkene as: \[{C_n}{H_{2n}}\]
We need to know that the electronic configuration of C in ground state: \[1{s^2}2{s^2}2{p^2}\]
Excitation of electron from s orbital to vacant p orbital so,
We must know that the electronic configuration of C in excited state: \[1{s^2}2{s^1}2{p^3}\]
Therefore, Hybridization of Ethene= \[s{p^2}\] one electron will be unpaired that is an unhybridized orbital and 3 \[s{p^2}\]orbitals.
We need to remember that the ethene has geometry is Planar
We can draw the structure of ethane molecule as,
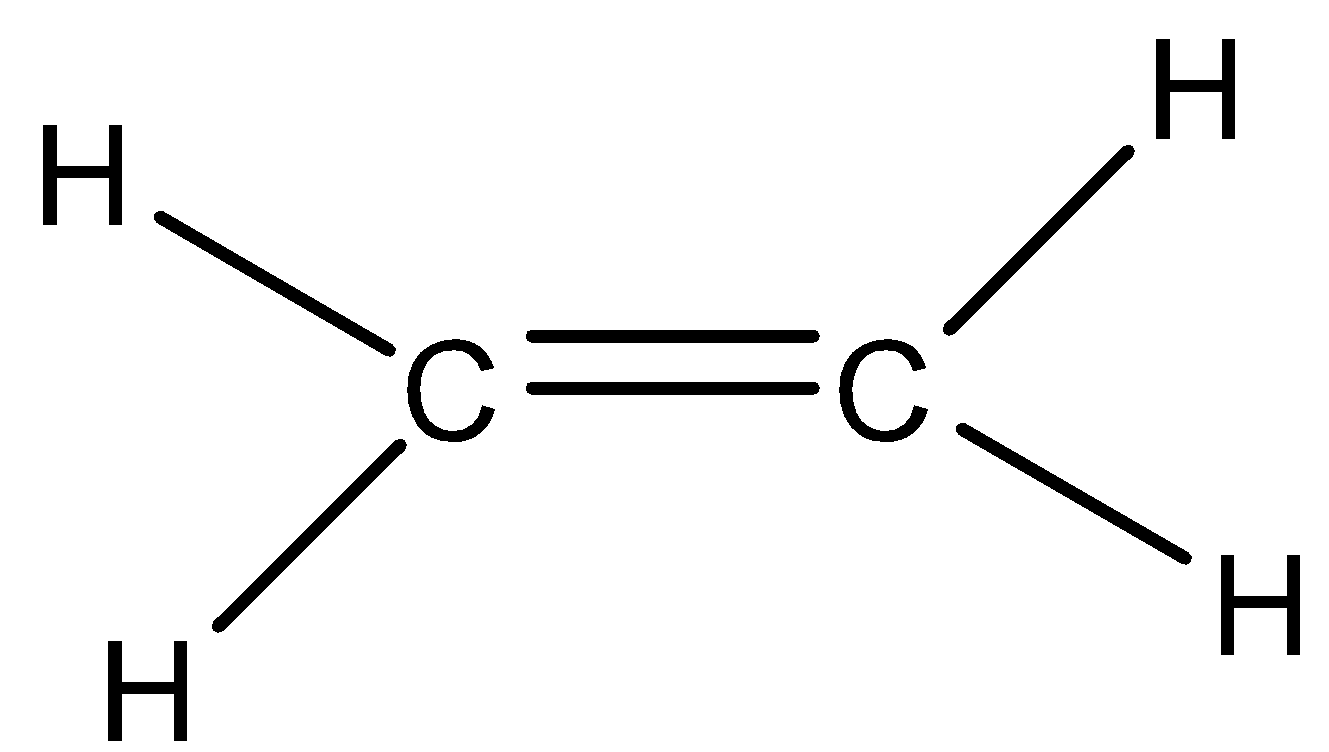
Option A) this is a correct option as the molecule has a planar shape as it has \[s{p^2}\] hybridization.
Option B) This is an incorrect option as tetrahedral geometry is for \[s{p^3}\] hybridization.
Option C) this is an incorrect option as bipyramidal geometry has a hybridization of \[s{p^3}d\].
Option D) this is an incorrect option as we got a correct option as ethene has planar shape.
Hence, the correct answer is, ‘Option A’.
Note: We need to remember that the ethane has molecular formula \[{C_2}{H_6}\]; Ethene has a molecular formula \[{C_2}{H_4}\] and ethylene has molecular formula \[{C_2}{H_2}\] having slightly sweet smell. We need to know that the ethylene is a nonpolar molecule and soluble in non-polar solvents.
Complete step by step answer:
We have to know that the ethene has a molecular formula \[{C_2}{H_4}\]. It has a double bond and therefore belongs to alkene.
We can write the general formula of Alkene as: \[{C_n}{H_{2n}}\]
We need to know that the electronic configuration of C in ground state: \[1{s^2}2{s^2}2{p^2}\]
Excitation of electron from s orbital to vacant p orbital so,
We must know that the electronic configuration of C in excited state: \[1{s^2}2{s^1}2{p^3}\]
Therefore, Hybridization of Ethene= \[s{p^2}\] one electron will be unpaired that is an unhybridized orbital and 3 \[s{p^2}\]orbitals.
We need to remember that the ethene has geometry is Planar
We can draw the structure of ethane molecule as,

Option A) this is a correct option as the molecule has a planar shape as it has \[s{p^2}\] hybridization.
Option B) This is an incorrect option as tetrahedral geometry is for \[s{p^3}\] hybridization.
Option C) this is an incorrect option as bipyramidal geometry has a hybridization of \[s{p^3}d\].
Option D) this is an incorrect option as we got a correct option as ethene has planar shape.
Hence, the correct answer is, ‘Option A’.
Note: We need to remember that the ethane has molecular formula \[{C_2}{H_6}\]; Ethene has a molecular formula \[{C_2}{H_4}\] and ethylene has molecular formula \[{C_2}{H_2}\] having slightly sweet smell. We need to know that the ethylene is a nonpolar molecule and soluble in non-polar solvents.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




