
What is the shape of $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule?
(A) Trigonal planar
(B) Trigonal pyramidal
(C) T-shaped
(D) Tetrahedral
Answer
572.1k+ views
Hint: The valence shell electron pair repulsion (VSEPR) theory is used to determine the shape of molecules. According to VSEPR theory the lone pair-lone pair has the maximum repulsion followed by the bond pair-lone pair and bond pair –bond pair. In $\text{ Cl}{{\text{F}}_{\text{3}}}\text{ }$ two lone pairs are placed in axial plane which minimises the repulsion and stabilises the molecule.
Complete Solution :
In$\text{ Cl}{{\text{F}}_{\text{3 }}}$, the chlorine is a central atom. The electronic configuration of chlorine atom is as below:
$\text{ Cl = 1}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{p}}^{\text{6 }}}\text{3}{{\text{s}}^{\text{2}}}\text{ 3}{{\text{p}}^{\text{5}}}\text{ }$
Chlorine atom has seven valence electrons. In $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule, the central chlorine atom is surrounded by three fluorine atoms. Each fluorine atom shares a one electron with chlorine atom and results in three covalent $\text{ Cl}-\text{F }$ bonds.
The $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule has 7 valence electrons from chlorine and three from Fluorine. Thus there are a total 10 electrons around the central chlorine atom.
Now let’s determine the electron pair in $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule. Divide total number of electrons by 2.
$\text{ Electron pair = }\dfrac{10}{2}=\text{ 5 }{{\text{e}}^{-\text{ }}}$
There are a total of 5 electron pairs in $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule. Let’s calculate the number of lone pairs in the $\text{ Cl}{{\text{F}}_{\text{3 }}}$molecule. The lone pair of electrons can be determined by subtraction of the number of bond pairs from the electron pair. As follows:
$\text{ Lone pair = Electron pair} - \text{Lone pair = 5} - \text{3 = 2 }$
Now we know that $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule has three bonding pairs and two lone pairs. Now according to VSEPR theory the lone pair –lone pair experience the maximum repulsion which can be reduced by placing two lone pairs in the axial plane. The shape of $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule is as shown below:
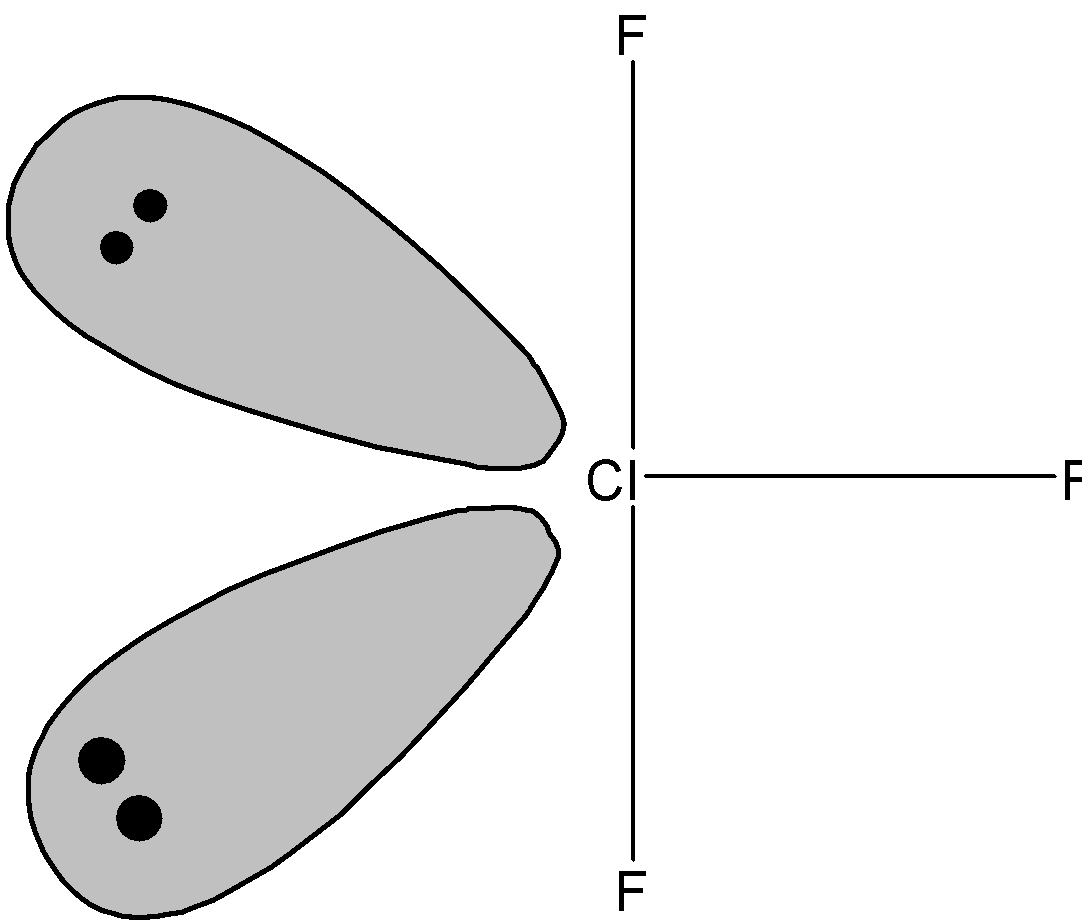
Therefore, $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule shows T-shaped geometry.
So, the correct answer is “Option C”.
Note: From local observation, we can say that $\text{ Cl}{{\text{F}}_{\text{3 }}}$ is a trigonal planar molecule. But, one should always consider the lone pair on the central atom. The number of lone pairs can be determined by a formula.
$\begin{align}
& \text{ No}\text{.of LP = BP + }\dfrac{1}{2}\left[ \text{Group attached}-\text{Valency }\pm \text{ Charge} \right] \\
& \Rightarrow \text{No}\text{.of LP }=\text{ 3 + }\dfrac{1}{2}\left[ 5-\text{7} \right] \\
& \therefore \text{No}\text{.of LP in Cl}{{\text{F}}_{\text{3}}}=\text{ 3 }-1\text{ = 2 L}\text{.P}\text{.} \\
\end{align}$
Complete Solution :
In$\text{ Cl}{{\text{F}}_{\text{3 }}}$, the chlorine is a central atom. The electronic configuration of chlorine atom is as below:
$\text{ Cl = 1}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{p}}^{\text{6 }}}\text{3}{{\text{s}}^{\text{2}}}\text{ 3}{{\text{p}}^{\text{5}}}\text{ }$
Chlorine atom has seven valence electrons. In $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule, the central chlorine atom is surrounded by three fluorine atoms. Each fluorine atom shares a one electron with chlorine atom and results in three covalent $\text{ Cl}-\text{F }$ bonds.
The $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule has 7 valence electrons from chlorine and three from Fluorine. Thus there are a total 10 electrons around the central chlorine atom.
Now let’s determine the electron pair in $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule. Divide total number of electrons by 2.
$\text{ Electron pair = }\dfrac{10}{2}=\text{ 5 }{{\text{e}}^{-\text{ }}}$
There are a total of 5 electron pairs in $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule. Let’s calculate the number of lone pairs in the $\text{ Cl}{{\text{F}}_{\text{3 }}}$molecule. The lone pair of electrons can be determined by subtraction of the number of bond pairs from the electron pair. As follows:
$\text{ Lone pair = Electron pair} - \text{Lone pair = 5} - \text{3 = 2 }$
Now we know that $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule has three bonding pairs and two lone pairs. Now according to VSEPR theory the lone pair –lone pair experience the maximum repulsion which can be reduced by placing two lone pairs in the axial plane. The shape of $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule is as shown below:

Therefore, $\text{ Cl}{{\text{F}}_{\text{3 }}}$ molecule shows T-shaped geometry.
So, the correct answer is “Option C”.
Note: From local observation, we can say that $\text{ Cl}{{\text{F}}_{\text{3 }}}$ is a trigonal planar molecule. But, one should always consider the lone pair on the central atom. The number of lone pairs can be determined by a formula.
$\begin{align}
& \text{ No}\text{.of LP = BP + }\dfrac{1}{2}\left[ \text{Group attached}-\text{Valency }\pm \text{ Charge} \right] \\
& \Rightarrow \text{No}\text{.of LP }=\text{ 3 + }\dfrac{1}{2}\left[ 5-\text{7} \right] \\
& \therefore \text{No}\text{.of LP in Cl}{{\text{F}}_{\text{3}}}=\text{ 3 }-1\text{ = 2 L}\text{.P}\text{.} \\
\end{align}$
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




