
How can we separate a mixture of two immiscible liquids? Describe the process.
Answer
532.5k+ views
Hint: There are two types of liquid mixtures i.e. miscible (two liquids are mixed evenly) and immiscible (two liquids do not mix properly; even after shaking they tend to separate after some time).
Complete answer:
Let us understand the process in detail;
Immiscible liquids –
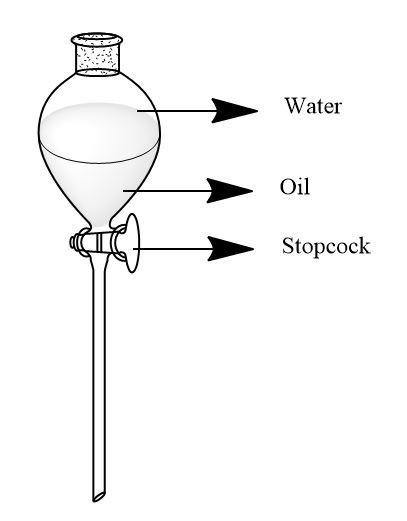
As the name suggests, the liquids are not miscible i.e. dissolving into each other. To take into note the most common example of two immiscible liquids is the mixture of oil and water.
When this mixture is created, the oil and water forms separate layers in the container. As oil is denser than that of water, it will form a layer at the bottom side of the container below the layer of water. The difference in their densities make the separation easy by just a separating funnel.
Separating funnel is a kind of funnel which has a stopcock at the nozzle point which can be operated to separate the mixture. We need to hold a beaker under the separating funnel and start the stopcock, the denser liquid will flow into the beaker. The less dense liquid which was at the upper side of the funnel will remain there when we stop the stopcock.
This is described firmly by following diagram-
Note:
Do note that the simple phenomenon of difference in densities is responsible for the separation of two immiscible liquids and thus, the method is simpler through the separating funnel.
Otherwise, for separation of two miscibles we need to take into account the system of fractional distillation which then separates according to the difference in volatilities.
Complete answer:
Let us understand the process in detail;
Immiscible liquids –
As the name suggests, the liquids are not miscible i.e. dissolving into each other. To take into note the most common example of two immiscible liquids is the mixture of oil and water.
When this mixture is created, the oil and water forms separate layers in the container. As oil is denser than that of water, it will form a layer at the bottom side of the container below the layer of water. The difference in their densities make the separation easy by just a separating funnel.
Separating funnel is a kind of funnel which has a stopcock at the nozzle point which can be operated to separate the mixture. We need to hold a beaker under the separating funnel and start the stopcock, the denser liquid will flow into the beaker. The less dense liquid which was at the upper side of the funnel will remain there when we stop the stopcock.
This is described firmly by following diagram-

Note:
Do note that the simple phenomenon of difference in densities is responsible for the separation of two immiscible liquids and thus, the method is simpler through the separating funnel.
Otherwise, for separation of two miscibles we need to take into account the system of fractional distillation which then separates according to the difference in volatilities.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




