
How will you separate a mixture of naphthalene balls powder and common salt? Draw a neat and labelled diagram to show that process.
Answer
513k+ views
Hint: Before going to the problem directly let us first get some idea about the mixture. A mixture is a substance composed of two or more distinct chemicals that are not chemically joined. A mixture is a physical mixing of two or more substances that retain their identities and are blended in the form of solutions, suspensions, or colloids.
Complete answer: The technique of extracting naphthalene from common salt is known as sublimation. Sublimation is the process of converting a solid into vapours without going through the liquid state. Sublimation is the process of a substance transitioning directly from a solid to a gas state without passing through a liquid stage. Sublimation is an endothermic process that occurs when a substance's triple point on its phase diagram, which corresponds to the lowest pressure at which the substance can exist as a liquid, is reached. Deposition or desublimation is the reversal of sublimation, in which a substance transitions from a gas to a solid state. The term "sublimation" has also been used to denote a solid-to-gas transition followed by a gas-to-solid transition.
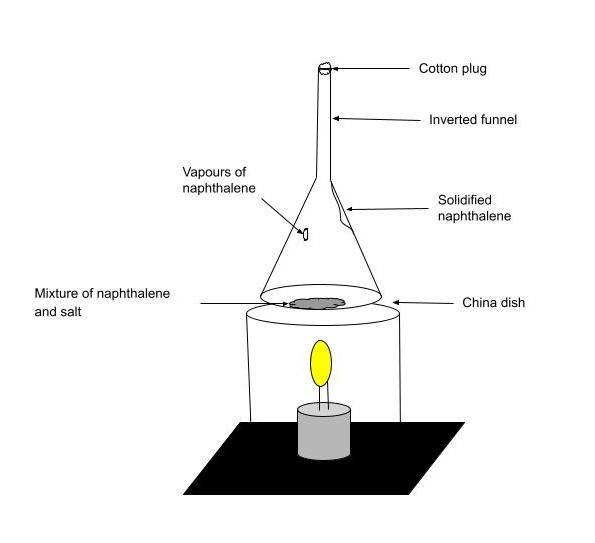
Naphthalene is a sublimating substance because it possesses weak intermolecular forces of attraction between its molecules and hence sublimes into a gaseous state without leaving any residue. After sublimation, it can be recovered, with the common salt remaining as a residue.
Note:
The absorption of heat provides enough energy for some molecules to overcome the attraction forces of their neighbours and escape into the vapour phase, resulting in sublimation. It's an endothermic transition because it necessitates more energy. By adding the enthalpy of fusion and the enthalpy of evaporation, the enthalpy of sublimation (also known as heat of sublimation) may be computed.
Complete answer: The technique of extracting naphthalene from common salt is known as sublimation. Sublimation is the process of converting a solid into vapours without going through the liquid state. Sublimation is the process of a substance transitioning directly from a solid to a gas state without passing through a liquid stage. Sublimation is an endothermic process that occurs when a substance's triple point on its phase diagram, which corresponds to the lowest pressure at which the substance can exist as a liquid, is reached. Deposition or desublimation is the reversal of sublimation, in which a substance transitions from a gas to a solid state. The term "sublimation" has also been used to denote a solid-to-gas transition followed by a gas-to-solid transition.

Naphthalene is a sublimating substance because it possesses weak intermolecular forces of attraction between its molecules and hence sublimes into a gaseous state without leaving any residue. After sublimation, it can be recovered, with the common salt remaining as a residue.
Note:
The absorption of heat provides enough energy for some molecules to overcome the attraction forces of their neighbours and escape into the vapour phase, resulting in sublimation. It's an endothermic transition because it necessitates more energy. By adding the enthalpy of fusion and the enthalpy of evaporation, the enthalpy of sublimation (also known as heat of sublimation) may be computed.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




