
Select the ether among the following that yields methanol as one of the products on reaction with cold hydroiodic acid.
A. 1-Methoxybutane
B. 1-Methoxy-2-methylpropane
C. 2- Methoxy-2-methylpropane
D. Methoxybenzene
Answer
603k+ views
Hint: This reaction is based on the concept of testing the presence of ethers in a chemical substance with the help of a mixture of acetic acid, and HI (hydrogen iodide, or hydroiodic acid). Write down the chemical reaction, and the end product could be known. The reaction between the ether, and HI to produce ethanol is termed as the Zeisel method.
Complete step by step answer:
As we know for unsymmetrical ethers if there is presence of tertiary groups, then the alkyl halide is formed from the smaller alkyl group, but if a tertiary group is present then the halogen gets attached to it.
We have to identify the ether, so let us talk about the given options. The first is 1-methoxybutane, it will form butanol, and methyl iodide when it reacts with HI.
So, we can write the reaction, i.e.
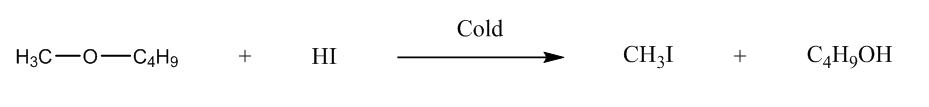
The second is 1-Methoxy-2-methylpropane, it will form methyl iodide, and tertiary alcohol.We can represent the reaction as:
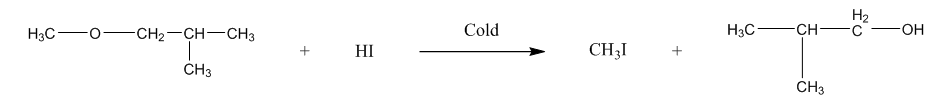
Now, the third is 2-Methoxy-2-methylpropane, it will form methanol, and tertiary halide. We can write the reaction as:
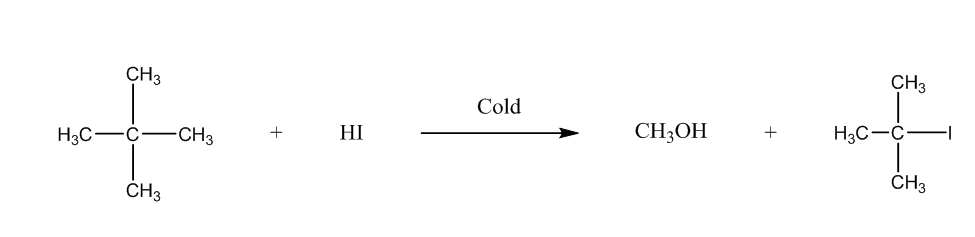
The fourth is methoxybenzene, it will form phenol, and methyl iodide. The chemical reaction is
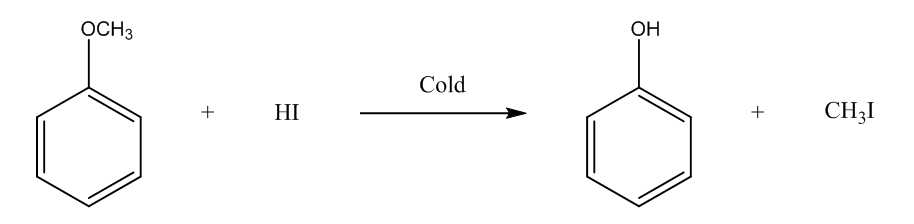
In the last, we can conclude that the 2-Methoxy-2-methylpropane ether will form methanol on reacting with cold HI.
Hence, the correct option is (C).
Note : As mentioned if a tertiary alkyl group is present, halogen will get attached to it. So in the third option there is formation of tertiary alkyl halide. In the fourth option there is benzene ring, so halogen is attached with methyl group leading to formation of methyl iodide and not methanol.
Complete step by step answer:
As we know for unsymmetrical ethers if there is presence of tertiary groups, then the alkyl halide is formed from the smaller alkyl group, but if a tertiary group is present then the halogen gets attached to it.
We have to identify the ether, so let us talk about the given options. The first is 1-methoxybutane, it will form butanol, and methyl iodide when it reacts with HI.
So, we can write the reaction, i.e.

The second is 1-Methoxy-2-methylpropane, it will form methyl iodide, and tertiary alcohol.We can represent the reaction as:

Now, the third is 2-Methoxy-2-methylpropane, it will form methanol, and tertiary halide. We can write the reaction as:

The fourth is methoxybenzene, it will form phenol, and methyl iodide. The chemical reaction is

In the last, we can conclude that the 2-Methoxy-2-methylpropane ether will form methanol on reacting with cold HI.
Hence, the correct option is (C).
Note : As mentioned if a tertiary alkyl group is present, halogen will get attached to it. So in the third option there is formation of tertiary alkyl halide. In the fourth option there is benzene ring, so halogen is attached with methyl group leading to formation of methyl iodide and not methanol.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




