
Select the correct statement about?
A. All C atoms are \[s{p^2}\] -hybridised
B. All C atoms are \[s{p^3}\]- hybridised
C. All C atoms are \[sp\] - hybridised
D. None of the above
Answer
564k+ views
Hint: Benzene is present as a cyclic structure contains six carbons and six hydrogens. The alternated double bond ( conjugated system) is present in the benzene ring. The structure of benzene was first determined by Kekule. Kekule had a dream about a snake seizing its tail and thus, after that, he confirms that benzene must possess a cyclic structure.
Complete step by step answer:
Kekule proposed a benzene structure. The structure of benzene can be drawn as,
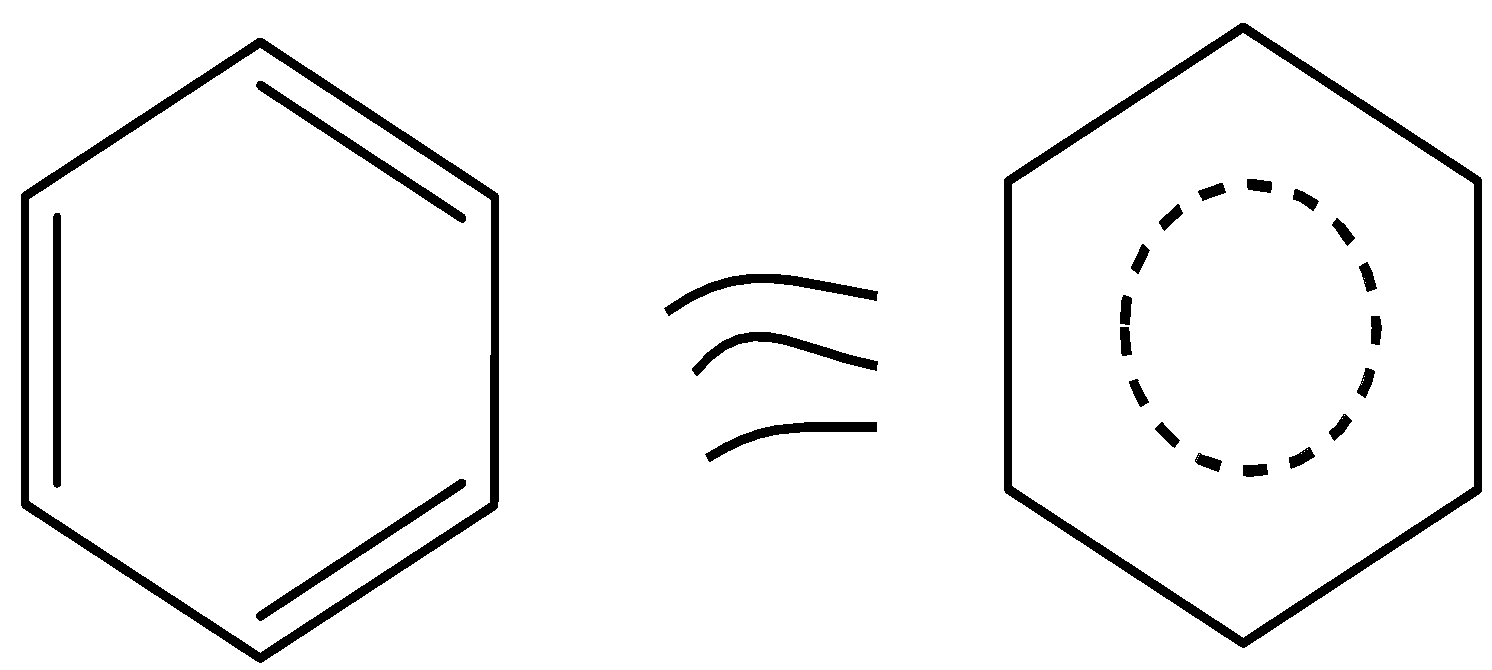
Thus, from that structure, it is clear that benzene contains six carbons and six hydrogens. Benzene alternately has three double bonds, thus it acts as a conjugate system. These characteristics define the aromaticity of the compound.
Benzene is involved in resonance and thus it exhibits maximum aromaticity when compared to other ring structures. The aromaticity of the compounds can be determined by Huckel’s \[(4n + 2)\pi {e^ - }\] rule where n $ = $ 1,2,3 ….
In a benzene ring, it has three double bonds and each double bond contains two electrons. Benzene has six electrons. When $n = 1$ in \[(4n + 2)\pi {e^ - }\] rule, then we get the value as 6 similar to several double-bonded electrons in benzene. Thus, benzene is aromatic since it obeys Huckel's rule.
All carbon in the benzene ring is \[s{p^2}\] -hybridised since each carbon benzene has \[3\sigma \] a bond and \[1\pi \]. Sigma bond defines its hybridisation. If \[\sigma = 3\] , then the hybridization must be \[s{p^2}\].
Thus, All C atoms in the benzene ring are \[s{p^2}\] -hybridised.
Thus, the correct answer is option C.
Note: Sigma bond refers to a single bond and pi-bond refers to a double bond. If \[\sigma = 2\], then the hybridization must be \[sp\]. The best example of \[sp\] hybridisation in ethyne. If \[\sigma = 4\], then the hybridization must be \[s{p^3}\]. The best example of \[s{p^3}\] hybridisation is methane.
Complete step by step answer:
Kekule proposed a benzene structure. The structure of benzene can be drawn as,

Thus, from that structure, it is clear that benzene contains six carbons and six hydrogens. Benzene alternately has three double bonds, thus it acts as a conjugate system. These characteristics define the aromaticity of the compound.
Benzene is involved in resonance and thus it exhibits maximum aromaticity when compared to other ring structures. The aromaticity of the compounds can be determined by Huckel’s \[(4n + 2)\pi {e^ - }\] rule where n $ = $ 1,2,3 ….
In a benzene ring, it has three double bonds and each double bond contains two electrons. Benzene has six electrons. When $n = 1$ in \[(4n + 2)\pi {e^ - }\] rule, then we get the value as 6 similar to several double-bonded electrons in benzene. Thus, benzene is aromatic since it obeys Huckel's rule.
All carbon in the benzene ring is \[s{p^2}\] -hybridised since each carbon benzene has \[3\sigma \] a bond and \[1\pi \]. Sigma bond defines its hybridisation. If \[\sigma = 3\] , then the hybridization must be \[s{p^2}\].
Thus, All C atoms in the benzene ring are \[s{p^2}\] -hybridised.
Thus, the correct answer is option C.
Note: Sigma bond refers to a single bond and pi-bond refers to a double bond. If \[\sigma = 2\], then the hybridization must be \[sp\]. The best example of \[sp\] hybridisation in ethyne. If \[\sigma = 4\], then the hybridization must be \[s{p^3}\]. The best example of \[s{p^3}\] hybridisation is methane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




