
Select the correct basic character:
A. \[NiO < MgO < SrO < {{K}_{2}}O < C{{s}_{2}}O\]
B. \[NiO < MgO < {{K}_{2}}O < BrO < C{{s}_{2}}O\]
C. \[MgO < NiO < SrO < {{K}_{2}}O < C{{s}_{2}}O\]
D. \[SrO < NiO < MgO < {{K}_{2}}O < C{{s}_{2}}O\]
Answer
608.4k+ views
Hint: To solve this question we should know that metal oxides are basic in character. We should focus on the trend of metallic character in periodic table that is Metallic character decreases as you move across a period in the periodic table from left to right and Metallic character increases as you move down an element group in the periodic table.
Step by step answer:
We know that there are trends in metallic character as we move across and down the periodic table. As we move across a period from left to right metallic character decreases. This occurs as atoms more readily accept electrons to fill a valence shell than lose them to remove the unfilled shell. And as we move down the element group metallic character increases. This is because electrons become easier to lose as the atomic radius increases, where there is less attraction between the nucleus and the valence electrons because of the increased distance between them.
We know that Metallic character is displayed by metals, which are all on the left-hand side of the periodic table. Elements with metallic character occur in certain groups or columns of elements, including the alkali metals, alkaline earth metals, transition metals (including the lanthanide and actinides below the main body of the periodic table), and the basic metals.
So if we look at our options, there are six oxides present. They are:
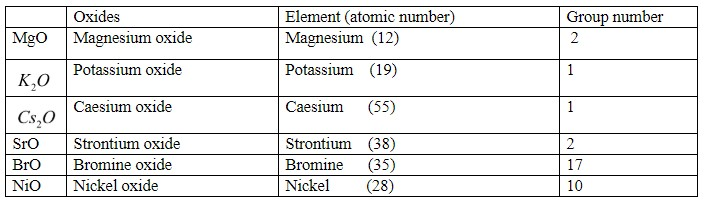
From the above table we can easily answer our question.
Metallic character increases down the group and from the above table, the highest atomic number is of caesium (55), so it has the highest metallic character. And that’s why; caesium oxide will show more basic nature.
After that, potassium oxide will be next because it is of group first and group first elements show more metallic character than elements of group 2. So potassium oxide will be taken next in order of basicity after caesium oxide. After that, strontium is in group 2 and it is below magnesium so it has high metallic character and basic character also. And magnesium is in group 2, so its oxide will be more metallic and show more basic character than nickel.
This is the correct order of increasing basic character.
So the correct order is shown by option A. That's why our correct answer is option A.
Note: We should know that metallic character is the name given to the set of chemical properties associated with elements that are metals. These chemical properties result from how readily metals lose their electrons to form cations (positively charged ions).
We can identify metal by physical properties that include metallic lustre, shiny appearance, high density, high thermal conductivity, and high electrical conductivity. Most metals are malleable and ductile and can be deformed without breaking. Although many metals are hard and dense, there is actually a wide range of values for these properties, even for elements that are considered highly metallic.
Step by step answer:
We know that there are trends in metallic character as we move across and down the periodic table. As we move across a period from left to right metallic character decreases. This occurs as atoms more readily accept electrons to fill a valence shell than lose them to remove the unfilled shell. And as we move down the element group metallic character increases. This is because electrons become easier to lose as the atomic radius increases, where there is less attraction between the nucleus and the valence electrons because of the increased distance between them.
We know that Metallic character is displayed by metals, which are all on the left-hand side of the periodic table. Elements with metallic character occur in certain groups or columns of elements, including the alkali metals, alkaline earth metals, transition metals (including the lanthanide and actinides below the main body of the periodic table), and the basic metals.
So if we look at our options, there are six oxides present. They are:

From the above table we can easily answer our question.
Metallic character increases down the group and from the above table, the highest atomic number is of caesium (55), so it has the highest metallic character. And that’s why; caesium oxide will show more basic nature.
After that, potassium oxide will be next because it is of group first and group first elements show more metallic character than elements of group 2. So potassium oxide will be taken next in order of basicity after caesium oxide. After that, strontium is in group 2 and it is below magnesium so it has high metallic character and basic character also. And magnesium is in group 2, so its oxide will be more metallic and show more basic character than nickel.
This is the correct order of increasing basic character.
So the correct order is shown by option A. That's why our correct answer is option A.
Note: We should know that metallic character is the name given to the set of chemical properties associated with elements that are metals. These chemical properties result from how readily metals lose their electrons to form cations (positively charged ions).
We can identify metal by physical properties that include metallic lustre, shiny appearance, high density, high thermal conductivity, and high electrical conductivity. Most metals are malleable and ductile and can be deformed without breaking. Although many metals are hard and dense, there is actually a wide range of values for these properties, even for elements that are considered highly metallic.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




