
Saccharin, an artificial sweetener is manufactured from:
A. Cellulose
B. Toluene
C. Cyclohexane
D. Starch
Answer
586.5k+ views
Hint: Try to recall that saccharin is an artificial sweetener which gives a sweetening effect to the food and enhances its odour and colour. Also, it is about 300-400 times as sweet as sucrose. Now, by using this you can easily solve the given question.
Complete step by step answer:
- We know that artificial sweeteners are the chemical substances which give sweetening effects to the food. Saccharin is a very popular artificial sweetener.
- The other name of saccharin is ortho-sulfabenzamide and its IUPAC name is 1,2-benzisothiazolin-3-one-1,1-dioxide. It is a white crystalline solid.
- It is excreted from the body in urine unchanged.
- It appears to be entirely inert and harmless when taken.
- It has a very sweet taste and is about 550 times sweeter than sucrose.
- The structure of saccharin is:
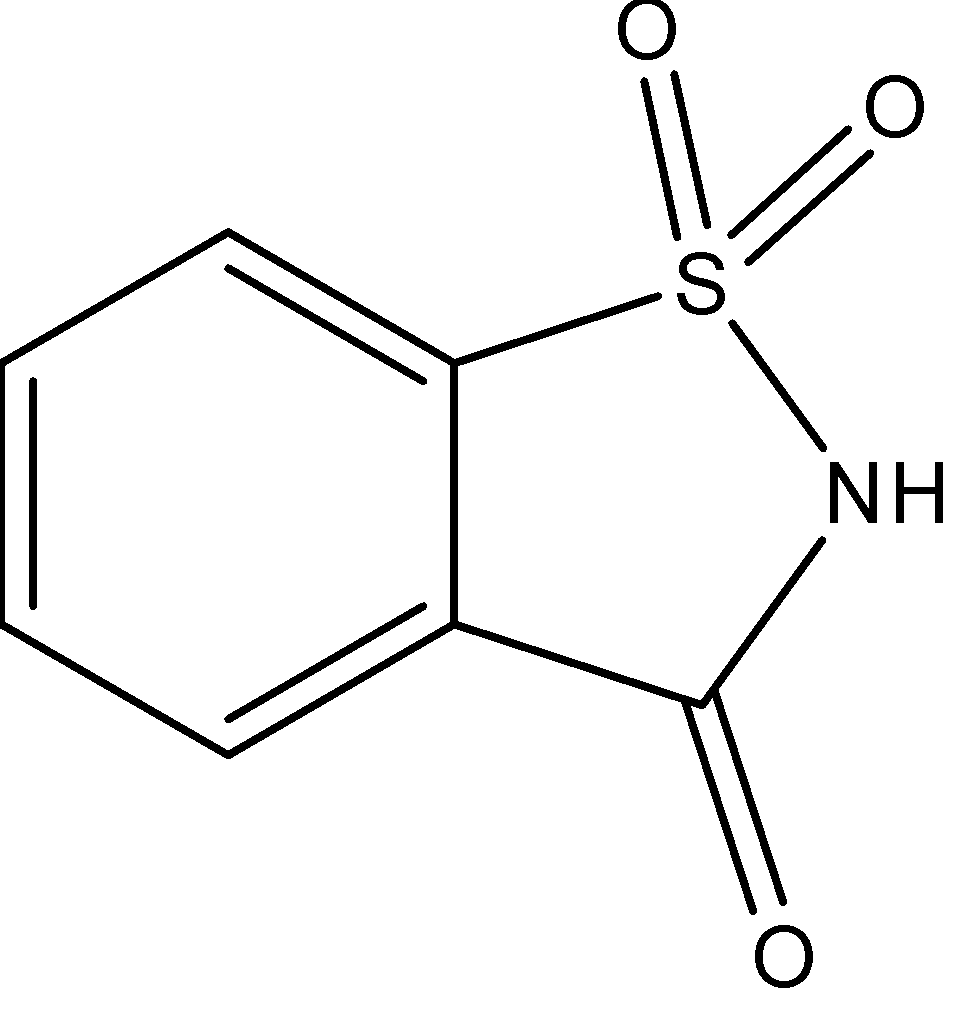
- It is prepared by sulfonation of toluene, followed by reaction with ammonia to form sulphonamide and then, oxidation of sulphonamide to form saccharin.
- The reaction for the formation of saccharin is as follows:
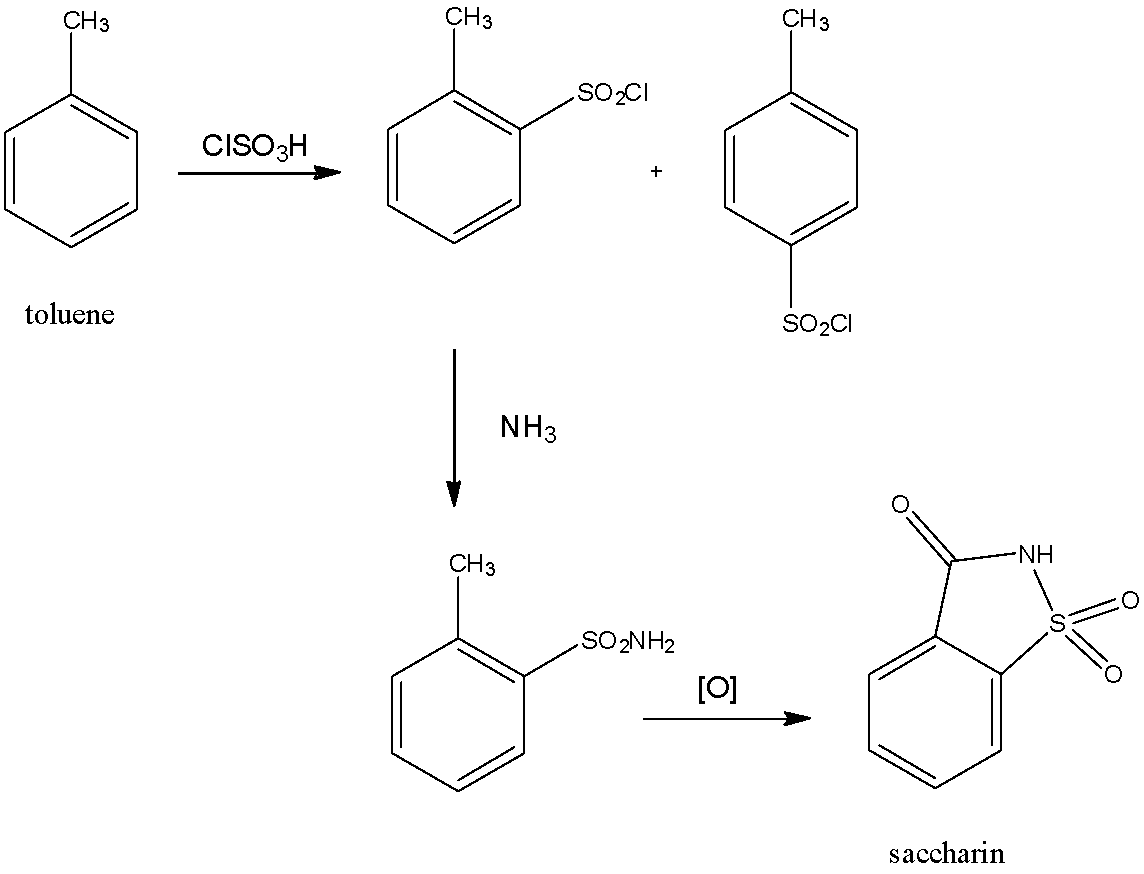
- The para isomer formed after the sulfonation of toluene is not considered since it cannot form saccharin.
- Also, sodium salt of saccharin is water soluble salt which is more palatable, and free from unpleasant after taste of saccharin.
So, the correct answer is “Option B”.
Note: Note that saccharin and its salts have been proved to be a lifesaver for countless diabetics and is of great value to people who need to control intake of calories. Also, you should remember that saccharin was the first discovered artificial sweetening agent.
Complete step by step answer:
- We know that artificial sweeteners are the chemical substances which give sweetening effects to the food. Saccharin is a very popular artificial sweetener.
- The other name of saccharin is ortho-sulfabenzamide and its IUPAC name is 1,2-benzisothiazolin-3-one-1,1-dioxide. It is a white crystalline solid.
- It is excreted from the body in urine unchanged.
- It appears to be entirely inert and harmless when taken.
- It has a very sweet taste and is about 550 times sweeter than sucrose.
- The structure of saccharin is:

- It is prepared by sulfonation of toluene, followed by reaction with ammonia to form sulphonamide and then, oxidation of sulphonamide to form saccharin.
- The reaction for the formation of saccharin is as follows:

- The para isomer formed after the sulfonation of toluene is not considered since it cannot form saccharin.
- Also, sodium salt of saccharin is water soluble salt which is more palatable, and free from unpleasant after taste of saccharin.
So, the correct answer is “Option B”.
Note: Note that saccharin and its salts have been proved to be a lifesaver for countless diabetics and is of great value to people who need to control intake of calories. Also, you should remember that saccharin was the first discovered artificial sweetening agent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




