
Resonance structures of aniline are shown below. Considering the above structures, it can be concluded that:
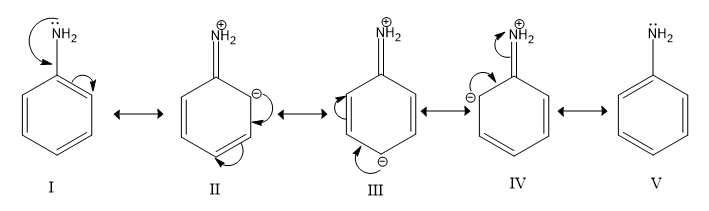
A.60% of the molecules of aniline exist as structure II, III, and IV
B. In aniline, the N-atom carries one unit positive charge
C. Aniline has a dipole moment with the N-atom as the positive end
D. The meta position of aniline is electron deficient in comparison to benzene

Answer
552.6k+ views
Hint: TLet us see how the resonance hybrid of Aniline looks like -
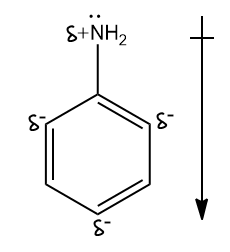
Now as you know the combination of all five structures, use the elimination method to find the answer.
Complete answer:
-Aniline is an aromatic amine in which the $-NH_2$ group is directly attached to hybridized carbon of benzene ring. Aniline exhibits a resonance effect due to which the lone pair on N-atoms participates in delocalization with electrons of the benzene ring system. So, these electrons are less available to be shared with other species.
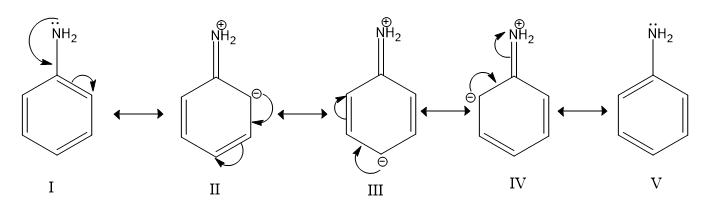
-All five structures exist equally in resonance because they contribute equally. So, option (a) is incorrect.
-In 3 (out of 5) resonance structures (II, III, IV) of aniline, the N atom has a positive charge (other N atoms are neutral) and one of the C atoms of the ring (ortho or para to the amino group) has a negative charge. Hence, in the resonance hybrid for aniline, the N atom has a partial positive charge and C atoms or the ring (ortho or para to the amino group) have a partial negative charge. So, we can conclude it denies option (b).
-Since N-atoms have a partial positive charge and carbon atoms in the benzene ring have a partial positive charge, the direction of dipole moment would be from AN-atom to the benzene ring, where N-atom is at the positive end. Hence, option (c) is correct.
-In resonance hybrid ortho and para, positions are more electron-dense due to partial negative charge on carbon atoms. There is no effect on the carbon at the meta position. Hence, the meta position of aniline is not electron deficient in comparison to benzene. That is why option (d) is incorrect.
Note:
Aniline is an organic chemical compound, specifically an aryl amine6, consisting of a phenyl group attached to an amino group. Chemically, aniline is a weak base. The aromatic amines like aniline are much weaker bases than aliphatic amines.

Now as you know the combination of all five structures, use the elimination method to find the answer.
Complete answer:
-Aniline is an aromatic amine in which the $-NH_2$ group is directly attached to hybridized carbon of benzene ring. Aniline exhibits a resonance effect due to which the lone pair on N-atoms participates in delocalization with electrons of the benzene ring system. So, these electrons are less available to be shared with other species.

-All five structures exist equally in resonance because they contribute equally. So, option (a) is incorrect.
-In 3 (out of 5) resonance structures (II, III, IV) of aniline, the N atom has a positive charge (other N atoms are neutral) and one of the C atoms of the ring (ortho or para to the amino group) has a negative charge. Hence, in the resonance hybrid for aniline, the N atom has a partial positive charge and C atoms or the ring (ortho or para to the amino group) have a partial negative charge. So, we can conclude it denies option (b).
-Since N-atoms have a partial positive charge and carbon atoms in the benzene ring have a partial positive charge, the direction of dipole moment would be from AN-atom to the benzene ring, where N-atom is at the positive end. Hence, option (c) is correct.
-In resonance hybrid ortho and para, positions are more electron-dense due to partial negative charge on carbon atoms. There is no effect on the carbon at the meta position. Hence, the meta position of aniline is not electron deficient in comparison to benzene. That is why option (d) is incorrect.
Note:
Aniline is an organic chemical compound, specifically an aryl amine6, consisting of a phenyl group attached to an amino group. Chemically, aniline is a weak base. The aromatic amines like aniline are much weaker bases than aliphatic amines.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Explain zero factorial class 11 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

State and prove Bernoullis theorem class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE




