
How many resonance structures are there for anthracene?
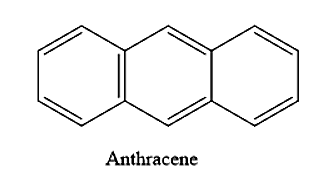
(A) 6
(B) 5
(C) 4
(D) 3

Answer
568.2k+ views
Hint: In order to give the number of resonance structures present in anthracene, we must first know about anthracene. Anthracene is the aromatic chemical compound with the chemical formula of \[{C_{14}}{H_{10}}\]. Anthracene will consist of three benzene rings which are fused together.
Complete answer:
Let us first understand about anthracene. Anthracene is an aromatic chemical compound which has a chemical formula of \[{C_{14}}{H_{10}}\]. Anthracene is made up of three benzene rings that are fused together. It is also known as the green oil or Para naphthalene. Anthracene can be present from the white to yellow solid. It will be having a very weak aromatic odour. Anthracene can be derived from the coal tar. It can be purified by the process of recrystallisation and sublimation. It can be identified in various places such as drinking water, exhaust emission, cigars and ambient air.
Now let us see what a resonance structure is. Resonance structure is the one or more Lewis structure of a polyatomic molecule which can show the delocalisation of the electron in a molecule. Sometimes it is helpful to determine the bonding in the molecule.
Let us see various resonance structures of Anthracene.
Anthracene will undergo delocalisation to give four resonance structures. The resonance structure of Anthracene is given below:
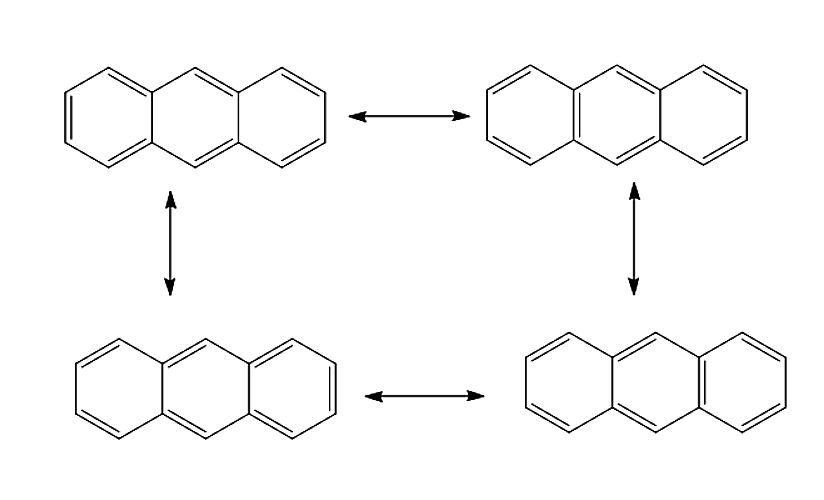
Therefore, the correct answer is option (C) 4.
Note:
Anthracene will be having various uses such as
- It can be used mainly in the production of dyes.
- Used in the organic semiconductor reaction.
- It is used in the scintillation counter crystal.
- Anthracene is converted into anthraquinone.
Complete answer:
Let us first understand about anthracene. Anthracene is an aromatic chemical compound which has a chemical formula of \[{C_{14}}{H_{10}}\]. Anthracene is made up of three benzene rings that are fused together. It is also known as the green oil or Para naphthalene. Anthracene can be present from the white to yellow solid. It will be having a very weak aromatic odour. Anthracene can be derived from the coal tar. It can be purified by the process of recrystallisation and sublimation. It can be identified in various places such as drinking water, exhaust emission, cigars and ambient air.
Now let us see what a resonance structure is. Resonance structure is the one or more Lewis structure of a polyatomic molecule which can show the delocalisation of the electron in a molecule. Sometimes it is helpful to determine the bonding in the molecule.
Let us see various resonance structures of Anthracene.
Anthracene will undergo delocalisation to give four resonance structures. The resonance structure of Anthracene is given below:

Therefore, the correct answer is option (C) 4.
Note:
Anthracene will be having various uses such as
- It can be used mainly in the production of dyes.
- Used in the organic semiconductor reaction.
- It is used in the scintillation counter crystal.
- Anthracene is converted into anthraquinone.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




