
What is the relationship between trans-2-butene and cis-2-butene?
A.Constitutional isomers
B.Diastereomers
C.Identical molecules
D.Enantiomers
Answer
575.7k+ views
Hint:According to the question isomers are the molecules having identical molecular formula but with different arrangement of atoms in space. Due to this they may have different chemical and physical properties.
Complete step by step answer:
Diastereomers are stereoisomers that are not mirror images of one another and are non-super imposable on one another.
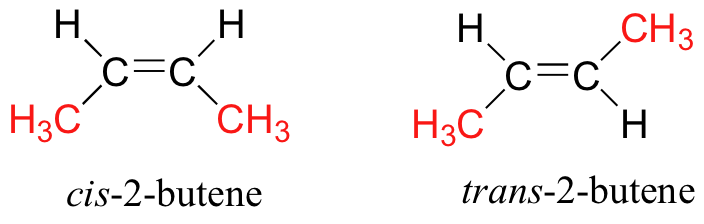
According to the diagram they are non super imposable on one another.cis-2- butene and trans-2- butene have similar chemical properties but different physical properties. Due to their different arrangements their properties change but their chemical formula is different. So according to the question cis-2-butene and trans-2-butene they are diastereomers.
Hence, option B is correct.
Note:
Isomers are the molecules having identical molecular formula but with different arrangement of atoms in space. According to their arrangement their properties may change. Enantiomers are molecules that are mirror images of one another but they are non super imposable on one another. Constitutional isomers are the molecules that have the same molecular formula but different connectivity. The more carbon atoms a hydrocarbon has, the greater the number of isomers.
There are different types of isomers so you should have clear knowledge about it. Due to their arrangement their chemical and physical properties may change. Clear concept about their structure and their formation.
Complete step by step answer:
Diastereomers are stereoisomers that are not mirror images of one another and are non-super imposable on one another.

According to the diagram they are non super imposable on one another.cis-2- butene and trans-2- butene have similar chemical properties but different physical properties. Due to their different arrangements their properties change but their chemical formula is different. So according to the question cis-2-butene and trans-2-butene they are diastereomers.
Hence, option B is correct.
Note:
Isomers are the molecules having identical molecular formula but with different arrangement of atoms in space. According to their arrangement their properties may change. Enantiomers are molecules that are mirror images of one another but they are non super imposable on one another. Constitutional isomers are the molecules that have the same molecular formula but different connectivity. The more carbon atoms a hydrocarbon has, the greater the number of isomers.
There are different types of isomers so you should have clear knowledge about it. Due to their arrangement their chemical and physical properties may change. Clear concept about their structure and their formation.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




