
Red P is less reactive, less volatile and less soluble in non-polar solvent than white/yellow P because:
A.It has high molecular energy
B.It has low molecular energy
C.It forms condensation products
D.It possesses high polymerised structure.
Answer
563.7k+ views
:Hint:Phosphorus is a chemical element which is highly reactive and thus, it is not found in Free State. It exists as red phosphorus and white phosphorus. It is an important element for the chemical balance and is also important for human bones and teeth and for guarding the carbohydrates and fats in our body.
Complete answer:
We know that phosphorus is a highly reactive chemical element found in two states that is red and white phosphorus. It is represented by the symbol P and has atomic number 15. It is very important for chemical balance and is also found in human bones and teeth. It also guards our body to use the carbohydrates and fats. It also helps the human body to make proteins which is highly important for human growth.
White phosphorus is P4 and has a tetrahedral geometry whereas red phosphorus is a polymerised structure of white/yellow P. We know that a polymerised structure is more stable and is less reactive and less volatile and less soluble in non-polar solvents. A polymerised structure is more stable as it has fewer hindrances. In P4 all the bonding of atoms is same and therefore, there is no polarity on each atom which results in no polarity on the whole compound
P4 White P
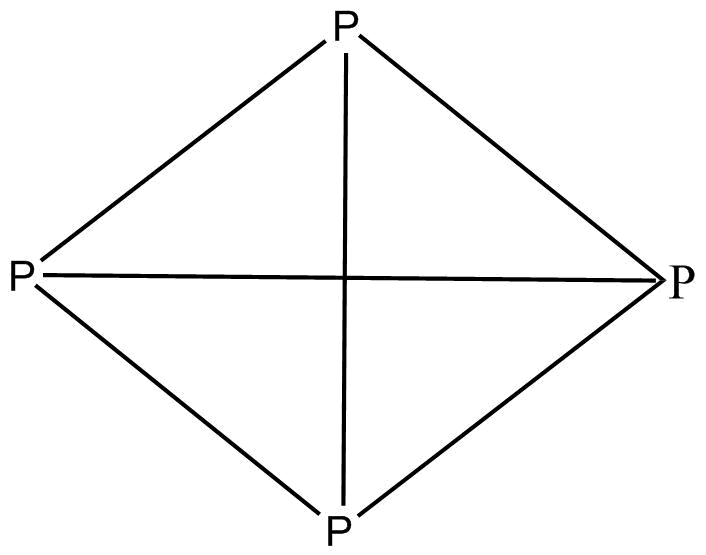
Red P
Thus we can say that Red P is less volatile, less reactive and less soluble in non-polar solvents as compare to white/yellow P as it is a polymerised structure of white/yellow P
Hence, the correct answer is option (D).
Note:
As phosphorus has large atomic size so it is not possible to form pi bonds and so it forms 3 sigma bonds with 3 phosphorus atoms as it is tetra-atomic. Phosphorus reacts with water to give a very harmful compound- phosphine which is highly toxic.
Complete answer:
We know that phosphorus is a highly reactive chemical element found in two states that is red and white phosphorus. It is represented by the symbol P and has atomic number 15. It is very important for chemical balance and is also found in human bones and teeth. It also guards our body to use the carbohydrates and fats. It also helps the human body to make proteins which is highly important for human growth.
White phosphorus is P4 and has a tetrahedral geometry whereas red phosphorus is a polymerised structure of white/yellow P. We know that a polymerised structure is more stable and is less reactive and less volatile and less soluble in non-polar solvents. A polymerised structure is more stable as it has fewer hindrances. In P4 all the bonding of atoms is same and therefore, there is no polarity on each atom which results in no polarity on the whole compound
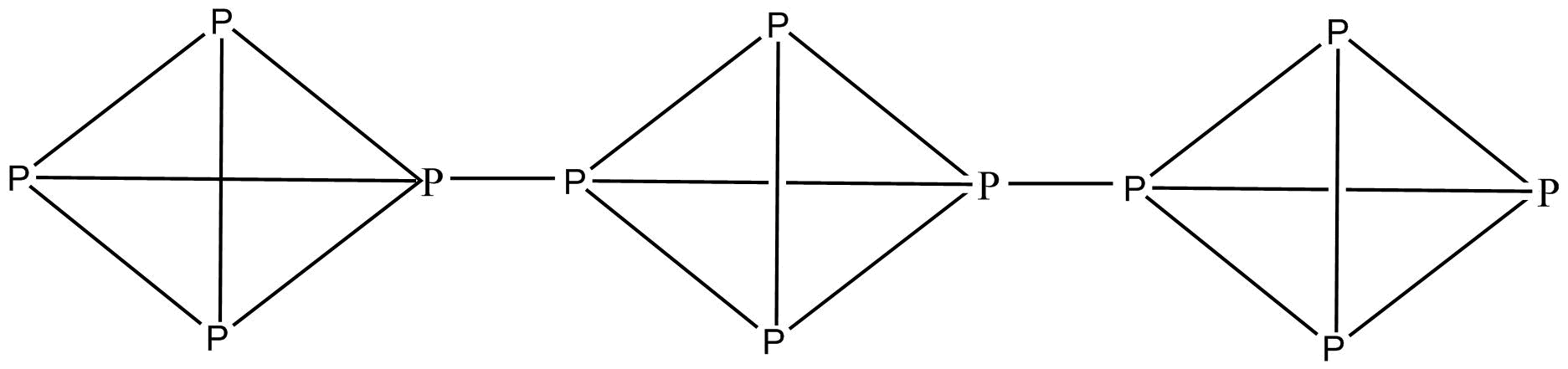
P4 White P

Red P

Thus we can say that Red P is less volatile, less reactive and less soluble in non-polar solvents as compare to white/yellow P as it is a polymerised structure of white/yellow P
Hence, the correct answer is option (D).
Note:
As phosphorus has large atomic size so it is not possible to form pi bonds and so it forms 3 sigma bonds with 3 phosphorus atoms as it is tetra-atomic. Phosphorus reacts with water to give a very harmful compound- phosphine which is highly toxic.
Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




