
Read the following statements:
I.When a litmus paper is dipped into a reaction mixture of a saponification reaction, it turns blue and the reaction is exothermic.
II.When a blue litmus paper is dipped into a reaction mixture of a saponification reaction, its color does not change and the reaction is exothermic.
III.When a red litmus paper is dipped into a reaction mixture of a saponification reaction, its color does not change and reaction is endothermic.
IV.When a blue litmus paper is dipped into a reaction mixture of a saponification reaction, its color does not change and the reaction is endothermic.
Which of the above statements are correct?
A.I and II
B.II and III
C.III and IV
D.I and IV
Answer
592.5k+ views
Hint: Saponification is a process to prepare soap from fat or lipid. It is an example of exothermic reaction because heat is going to liberate during the saponification process. The saponification process with soap glycerol is also going to be released as a product.
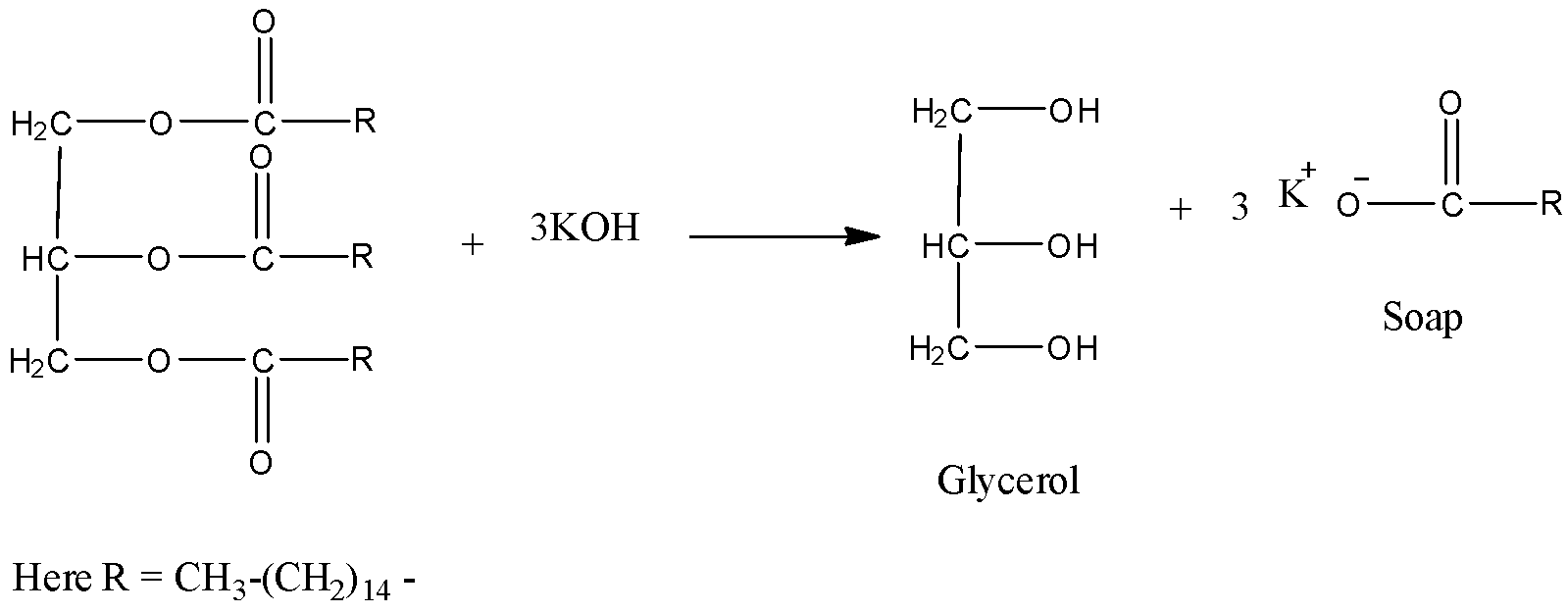
Complete answer:
We know that generally soap is basic in nature.
By using red litmus paper we can easily find the presence of basic compounds.
Basic compound turns red litmus paper into blue litmus paper.
Coming to given options, option I, when a litmus paper is dipped into a reaction mixture of a saponification reaction, it turns blue and the reaction is exothermic. Option I is correct as we discussed saponification is an exothermic process and prepares soap. Soap is basic in nature so it turns red litmus to blue color.
Coming to option II, when a blue litmus paper is dipped into a reaction mixture of a saponification reaction, its color does not change and the reaction is exothermic. Option II is also correct because blue litmus paper does not change its color in basic compounds.
Coming to option III, when a red litmus paper is dipped into a reaction mixture of a saponification reaction, its color does not change and reaction is endothermic. It is wrong because red litmus should change its color when it is dipped in saponification reaction mixture.
Coming to option IV, when a blue litmus paper is dipped into a reaction mixture of a saponification reaction, its color does not change and the reaction is endothermic. Saponification is an exothermic process. So, this option is wrong.
Therefore option I and Option II are correct.
So, the correct answer is option A.
Note:
Blue litmus paper used to test the presence of acidic compounds. If we are going to dip blue litmus paper in acidic solution the color of the blue litmus paper is going to change into red color. This test is very useful to determine the presence of acidic compounds in our daily life.

Complete answer:
We know that generally soap is basic in nature.
By using red litmus paper we can easily find the presence of basic compounds.
Basic compound turns red litmus paper into blue litmus paper.
Coming to given options, option I, when a litmus paper is dipped into a reaction mixture of a saponification reaction, it turns blue and the reaction is exothermic. Option I is correct as we discussed saponification is an exothermic process and prepares soap. Soap is basic in nature so it turns red litmus to blue color.
Coming to option II, when a blue litmus paper is dipped into a reaction mixture of a saponification reaction, its color does not change and the reaction is exothermic. Option II is also correct because blue litmus paper does not change its color in basic compounds.
Coming to option III, when a red litmus paper is dipped into a reaction mixture of a saponification reaction, its color does not change and reaction is endothermic. It is wrong because red litmus should change its color when it is dipped in saponification reaction mixture.
Coming to option IV, when a blue litmus paper is dipped into a reaction mixture of a saponification reaction, its color does not change and the reaction is endothermic. Saponification is an exothermic process. So, this option is wrong.
Therefore option I and Option II are correct.
So, the correct answer is option A.
Note:
Blue litmus paper used to test the presence of acidic compounds. If we are going to dip blue litmus paper in acidic solution the color of the blue litmus paper is going to change into red color. This test is very useful to determine the presence of acidic compounds in our daily life.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




