
Ratio of hydrogen and oxygen of water molecules.
A. 1:2
B. 2:3
C. 2:1
D. 3:2
Answer
600.9k+ views
Hint: It is already known to us that the chemical formula of water is and the ratio of hydrogen to oxygen atoms will always remain the same, no matter how many number of water molecules are taken.
Complete step by step solution:
Whenever $H_2O$ is produced by combining hydrogen and oxygen gas, then it always takes two molecules of diatomic hydrogen gas and one molecule of oxygen gas to produce two molecules of water. The chemical reaction which takes place is:
$
2\mathop H\nolimits_2 + \mathop O\nolimits_2 \to 2\mathop H\nolimits_2 O \\
$
In other words, from the above chemical reaction, we can say that the ratio of hydrogen to oxygen is 2:1, the ratio of hydrogen to water is 1:1 and the ratio of oxygen to water is 1:2.
Therefore, from above it can be easily said that option C is the correct answer.
Additional Information:
The hybridization of $H_2O$ molecule is $sp^3 $ and from hybridization we can easily say that the geometry of $H_2O$ molecule is tetrahedral and the structure of $H_2O$ is bent shape in gaseous phase. The geometry of water is
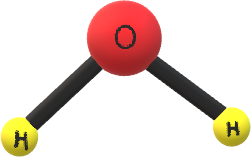
Also, water has a higher specific heat, thermal conductivity and surface tension compared to other liquids.
Also, natural water contains many dissolved salts and depending upon their behavior towards soap solution i.e. lather formation, they are classified as soft water and hot water.
Note: It should be kept in mind that most of the unique properties of water are due to the presence of hydrogen bonding between its molecules.
Also, it should be remembered that water is amphoteric in character i.e. can act both as an acid and as a base.
Complete step by step solution:
Whenever $H_2O$ is produced by combining hydrogen and oxygen gas, then it always takes two molecules of diatomic hydrogen gas and one molecule of oxygen gas to produce two molecules of water. The chemical reaction which takes place is:
$
2\mathop H\nolimits_2 + \mathop O\nolimits_2 \to 2\mathop H\nolimits_2 O \\
$
In other words, from the above chemical reaction, we can say that the ratio of hydrogen to oxygen is 2:1, the ratio of hydrogen to water is 1:1 and the ratio of oxygen to water is 1:2.
Therefore, from above it can be easily said that option C is the correct answer.
Additional Information:
The hybridization of $H_2O$ molecule is $sp^3 $ and from hybridization we can easily say that the geometry of $H_2O$ molecule is tetrahedral and the structure of $H_2O$ is bent shape in gaseous phase. The geometry of water is

Also, water has a higher specific heat, thermal conductivity and surface tension compared to other liquids.
Also, natural water contains many dissolved salts and depending upon their behavior towards soap solution i.e. lather formation, they are classified as soft water and hot water.
Note: It should be kept in mind that most of the unique properties of water are due to the presence of hydrogen bonding between its molecules.
Also, it should be remembered that water is amphoteric in character i.e. can act both as an acid and as a base.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




