
Who proposed the planetary model of the atom?
A. John Dalton (early ${\rm{180}}{{\rm{0}}^{\rm{'}}}{\rm{s}}$)
B. Ernest Rutherford\[\left( {1911} \right)\]
C. Niels Bohr$\left( {1913} \right)$
D. Joseph John Thomas$\left( {1897} \right)$
Answer
585k+ views
Hint: We know that, according to the planetary model of an atom, nucleus is positively charged due to the presence of protons in it and the negatively charged electrons revolve around it in a fixed circular orbit like planets spin around the sun. Therefore, this model is called the planetary model of the atom.
Complete step by step answer:
We know that, In $1808$, John Dalton proposed Dalton’s Atomic theory. Dalton’s theory states that every matter is composed of atoms which are indivisible and indestructible.
An atom is composed of three component particles which are electrons, protons and neutrons. The protons and neutrons are situated in the nucleus of an atom. However, the electrons are distributed uniformly outside the nucleus. There is no overall charge on an atom. This theory was proposed by Ernest Rutherford. Let’s discuss it in detail.
According to Rutherford, an atom is composed of a nucleus which is positively charged and the negatively charged electrons revolve around it in a fixed circular orbit. This conclusion was put forward on the basis of his scattering experiment.
The experiment performed by Rutherford gave three most important observations about an atom. The first observations of the alpha particle scattering experiment explains that most of the alpha particles passed straight from the foil without any failure in their direction. This observation confirms that there must be sufficient free space within the atom.
The second observation states that most of the alpha particles undergo deflection. Some particles experience small deflection whereas some particles experience large deflection. This observation confirms that there must be a positively charged body in an atom due to which the positively charged alpha particles experience repulsive force which lead to the small and large deflection. This confirms the presence of a heavy positively charged nucleus in the center of the atom.
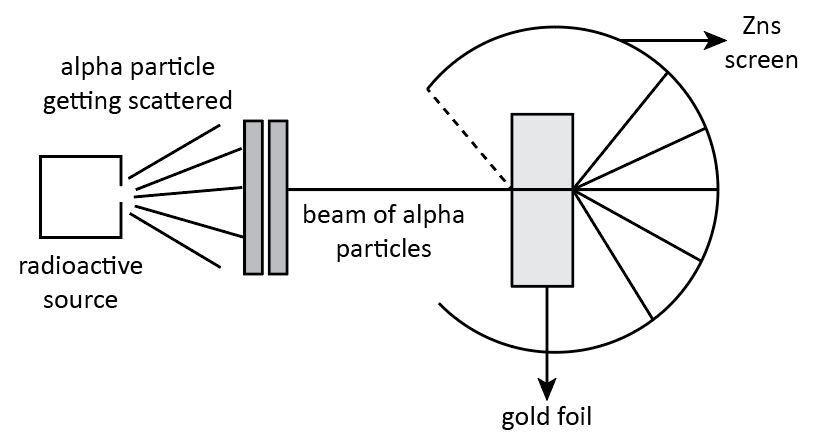
Hence, we can say that Ernest Rutherford proposed the planetary model of the atom.
Therefore, the correct option is option (B).
Note:
As we know that, the mass and properties of all the atoms present in an element are identical. After the discovery of atoms, the different subatomic particles were also discovered by various experiments done by scientists. In the year, $1911$, Ernest Rutherford describes the structure of an atom. This model is called the planetary model of the atom.
Complete step by step answer:
We know that, In $1808$, John Dalton proposed Dalton’s Atomic theory. Dalton’s theory states that every matter is composed of atoms which are indivisible and indestructible.
An atom is composed of three component particles which are electrons, protons and neutrons. The protons and neutrons are situated in the nucleus of an atom. However, the electrons are distributed uniformly outside the nucleus. There is no overall charge on an atom. This theory was proposed by Ernest Rutherford. Let’s discuss it in detail.
According to Rutherford, an atom is composed of a nucleus which is positively charged and the negatively charged electrons revolve around it in a fixed circular orbit. This conclusion was put forward on the basis of his scattering experiment.
The experiment performed by Rutherford gave three most important observations about an atom. The first observations of the alpha particle scattering experiment explains that most of the alpha particles passed straight from the foil without any failure in their direction. This observation confirms that there must be sufficient free space within the atom.
The second observation states that most of the alpha particles undergo deflection. Some particles experience small deflection whereas some particles experience large deflection. This observation confirms that there must be a positively charged body in an atom due to which the positively charged alpha particles experience repulsive force which lead to the small and large deflection. This confirms the presence of a heavy positively charged nucleus in the center of the atom.

Hence, we can say that Ernest Rutherford proposed the planetary model of the atom.
Therefore, the correct option is option (B).
Note:
As we know that, the mass and properties of all the atoms present in an element are identical. After the discovery of atoms, the different subatomic particles were also discovered by various experiments done by scientists. In the year, $1911$, Ernest Rutherford describes the structure of an atom. This model is called the planetary model of the atom.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




