
What products would you obtain from acidic and basic hydrolysis of the N,N-diethyl benzamide?
Answer
510.9k+ views
Hint: N,N-diethyl benzamide is a compound with the amide functional group. N,N-diethyl benzamide has two ethyl groups that form different products in case of acidic and basic hydrolysis. Hydrolysis is the process of cleaving the bonds by using water molecules that lead to different products.
Complete answer:
Given compound is N,N-diethyl benzamide .Generally, the amide group has one carbonyl group and nitrogen with two hydrogen atoms. But in case of N,N-diethyl benzamide the two hydrogen atoms in the amide group will be replaced by two ethyl groups.
Thus, the structure of N,N-diethyl benzamide will be
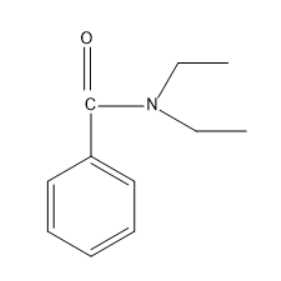
Hydrolysis are of two types basic hydrolysis and acidic hydrolysis.
In case of acidic hydrolysis, the hydronium ion will be added on N,N-diethyl benzamide to form the below products.
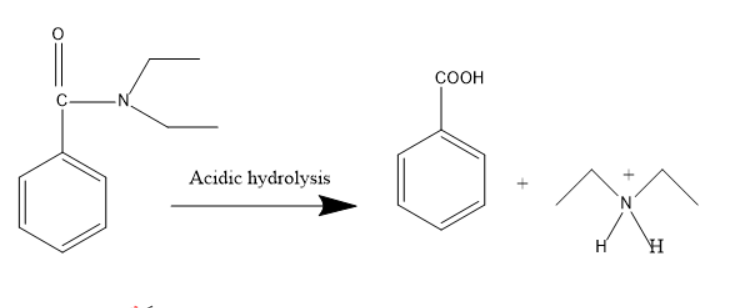
The products were benzoic acid and diethyl amine ion.
In case of basic hydrolysis, the products formed will be as follows:
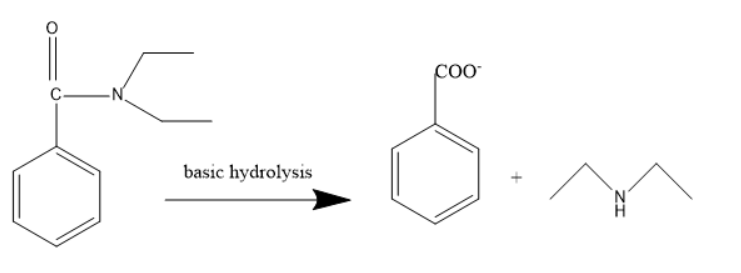
Incase of acidic hydrolysis an acid is formed with the positive ion and in case of basic hydrolysis the diethylamine and carboxylate ion is formed.
Note:
In both the cases the hydrolysis was done the action of water molecules only takes place. In acidic hydrolysis, hydronium ion can be used, in basic hydrolysis hydroxide ion can be used. The hydronium ion has the formula of \[{H_3}{O^ + }\] and the hydroxide ion has the formula of \[O{H^ - }\]. Both these are obtained from water molecules. Thus, though both these are different the process is known as hydrolysis.
Complete answer:
Given compound is N,N-diethyl benzamide .Generally, the amide group has one carbonyl group and nitrogen with two hydrogen atoms. But in case of N,N-diethyl benzamide the two hydrogen atoms in the amide group will be replaced by two ethyl groups.
Thus, the structure of N,N-diethyl benzamide will be

Hydrolysis are of two types basic hydrolysis and acidic hydrolysis.
In case of acidic hydrolysis, the hydronium ion will be added on N,N-diethyl benzamide to form the below products.

The products were benzoic acid and diethyl amine ion.
In case of basic hydrolysis, the products formed will be as follows:

Incase of acidic hydrolysis an acid is formed with the positive ion and in case of basic hydrolysis the diethylamine and carboxylate ion is formed.
Note:
In both the cases the hydrolysis was done the action of water molecules only takes place. In acidic hydrolysis, hydronium ion can be used, in basic hydrolysis hydroxide ion can be used. The hydronium ion has the formula of \[{H_3}{O^ + }\] and the hydroxide ion has the formula of \[O{H^ - }\]. Both these are obtained from water molecules. Thus, though both these are different the process is known as hydrolysis.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




