
What product would you obtain from acidic and basic hydrolysis of the N,N-diethylbenzamide?
Answer
502.8k+ views
Hint: Amides can be hydrolyzed in either acidic or basic solution, the mechanisms are much like those of ester hydrolysis, but the reactions are much slower. Hydrolysis under acidic conditions requires strong acids.
Complete answer:
Acidic hydrolysis:
After acidic hydrolysis, N,N-diethylbenzamide gives the desired products, after the addition of water, benzoic acid is formed.
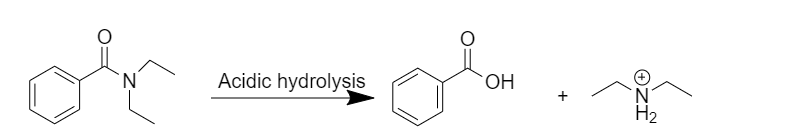
Hydrolysis under acidic conditions requires acid like hydrochloric acid, and temperature of about for several hours. The mechanism involves protonation of the amide on oxygen followed by an attack of water on the carbonyl carbon. The tetrahedral intermediate formed dissociates ultimately to the benzoic acid and protonated diethylamine.
Basic hydrolysis:
After basic hydrolysis, N,N-diethylbenzamide gives the desired products. Here, a benzoate ion is formed during the reaction.
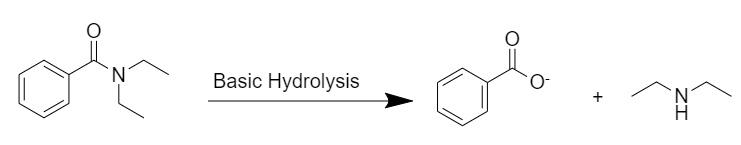
In basic hydrolysis the amide is heated with boiling aqueous sodium or potassium hydroxide. The nucleophile hydroxide ion adds to the carbonyl carbon to form a tetrahedral intermediate, which with the help of the aqueous solvent, expels the salt of benzoic acid and diethylamine.
Note:
Amides are not readily oxidized or reduced, although hydrogenation (addition of hydrogen at high temperature) in the presence of a catalyst will convert most amides into carboxylic acid to amines. The powerful reducing agent lithium aluminum hydride transforms amides into amines.
Complete answer:
Acidic hydrolysis:
After acidic hydrolysis, N,N-diethylbenzamide gives the desired products, after the addition of water, benzoic acid is formed.

Hydrolysis under acidic conditions requires acid like hydrochloric acid, and temperature of about for several hours. The mechanism involves protonation of the amide on oxygen followed by an attack of water on the carbonyl carbon. The tetrahedral intermediate formed dissociates ultimately to the benzoic acid and protonated diethylamine.
Basic hydrolysis:
After basic hydrolysis, N,N-diethylbenzamide gives the desired products. Here, a benzoate ion is formed during the reaction.

In basic hydrolysis the amide is heated with boiling aqueous sodium or potassium hydroxide. The nucleophile hydroxide ion adds to the carbonyl carbon to form a tetrahedral intermediate, which with the help of the aqueous solvent, expels the salt of benzoic acid and diethylamine.
Note:
Amides are not readily oxidized or reduced, although hydrogenation (addition of hydrogen at high temperature) in the presence of a catalyst will convert most amides into carboxylic acid to amines. The powerful reducing agent lithium aluminum hydride transforms amides into amines.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




