
What is the product of the following reaction?
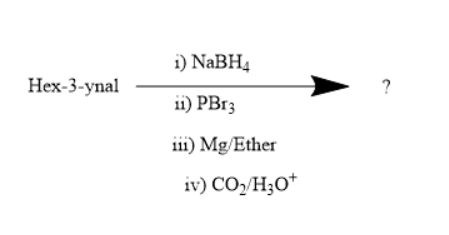

Answer
510.6k+ views
Hint: Alkynes are unsaturated hydrocarbons consisting of triple bonds and aldehydes belong to carbonyl compounds. When aldehydes treated with reducing agents like sodium borohydride form alcohols. Alcohols undergo nucleophilic substitution by replacing the hydroxyl group. Alkyl halides react with metal forms Grignard reagent and Grignard reagent on carboxylation forms carboxylic acids.
Complete answer:
Given molecule is Hex-3-ynal which means it consists of alkyne and aldehyde. The suffix -al represents aldehyde functional group and -yne represents alkyne functional group. The root word Hex represents six carbon atoms. The number \[3\] indicates the position of alkyne.
When Hex-3-ynal reacts with sodium borohydride, the aldehyde converts into alcohol and now this alcohol when treated with tribromo phosphine which is a bromide source, the hydroxyl group can be replaced by bromine forms bromo alkyne. When the bromo alkyne is treated with magnesium in presence of ether it forms Grignard reagent. When Grignard reagent is treated with carbon dioxide in presence of hydronium ion it forms carboxylic acid.
\[C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - CHO\xrightarrow{{NaB{H_4}}}C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - C{H_2}OH\]
When the above compound is treated with tribromo phosphine, it forms
\[C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - C{H_2}OH\xrightarrow{{PB{r_3}}}C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - C{H_2}Br\]
Further, it forms
\[C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - C{H_2}Br\xrightarrow{{Mg/ether}}C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - C{H_2}MgBr\]
The final product will be
\[C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - C{H_2}MgBr\xrightarrow{{C{O_2}/{H_3}{O^ + }}}C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - C{H_2}COOH\]
Thus, the product will be \[Hex - 3 - yne1 - oic acid\]
At the position \[3\], triple bond is present, \[{1^{st}}\] position carboxylic acid is present.
Note:
Sodium borohydride can be used as a reducing agent, it belongs to the hydrides family, as a source of hydrides used for the reduction of aldehydes to alcohols. Tribromo phosphine is the source of bromine, the magnesium when treated with bromine forms Grignard reagent. Grignard reagent undergoes carboxylation and forms carboxylic acids.
Complete answer:
Given molecule is Hex-3-ynal which means it consists of alkyne and aldehyde. The suffix -al represents aldehyde functional group and -yne represents alkyne functional group. The root word Hex represents six carbon atoms. The number \[3\] indicates the position of alkyne.
When Hex-3-ynal reacts with sodium borohydride, the aldehyde converts into alcohol and now this alcohol when treated with tribromo phosphine which is a bromide source, the hydroxyl group can be replaced by bromine forms bromo alkyne. When the bromo alkyne is treated with magnesium in presence of ether it forms Grignard reagent. When Grignard reagent is treated with carbon dioxide in presence of hydronium ion it forms carboxylic acid.
\[C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - CHO\xrightarrow{{NaB{H_4}}}C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - C{H_2}OH\]
When the above compound is treated with tribromo phosphine, it forms
\[C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - C{H_2}OH\xrightarrow{{PB{r_3}}}C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - C{H_2}Br\]
Further, it forms
\[C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - C{H_2}Br\xrightarrow{{Mg/ether}}C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - C{H_2}MgBr\]
The final product will be
\[C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - C{H_2}MgBr\xrightarrow{{C{O_2}/{H_3}{O^ + }}}C{H_3} - C{H_2} - C \equiv C - C{H_2} - C{H_2} - C{H_2}COOH\]
Thus, the product will be \[Hex - 3 - yne1 - oic acid\]
At the position \[3\], triple bond is present, \[{1^{st}}\] position carboxylic acid is present.
Note:
Sodium borohydride can be used as a reducing agent, it belongs to the hydrides family, as a source of hydrides used for the reduction of aldehydes to alcohols. Tribromo phosphine is the source of bromine, the magnesium when treated with bromine forms Grignard reagent. Grignard reagent undergoes carboxylation and forms carboxylic acids.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




