
What is the product of the following reaction?
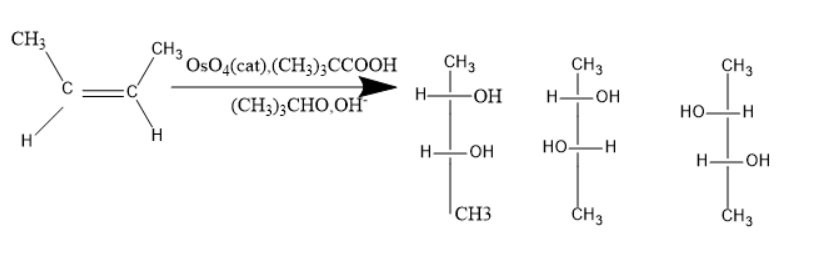
(A) Only $ 1 $
(B) $ 1:1 $ mixture of $ 2 $ and $ 3 $
(C) Only $ 2 $
(D) $ 1:1:1 $ mixture of $ 1,2, $ and $ 3 $

Answer
504.6k+ views
Hint: Alkenes are unsaturated hydrocarbons containing double bonds. Due to the presence of double bond alkenes undergo additional reactions, the reagent or molecules can be added on the double bond of alkenes to form new compounds. Alkenes treated with osmium tetroxide and acetic acid derivatives form syn diols.
Complete answer:
Alkenes consist of double bonds, the general molecular formula of alkenes is $ {C_n}{H_{2n}} $ , where n is the number of carbon atoms. Due to the presence of a double bond, the carbon in alkenes has $ s{p^2} $ hybridisation. Alkenes participate in additional reactions due to the presence of double bonds.
Osmium tetroxide with the combination of hydrogen peroxide or tri methyl acetic acid used as a material for the synthesis of $ 1,2 $ diols from alkenes.
Given molecules is $ 2 - butene $ when treated with osmium tetroxide and tri methyl acetic acid, the syn addition of hydroxyl groups takes place. Thus, the diols formed will be syn-diols.
In the given reaction, only one product is syn diol. The syn diol has two hydroxyl groups on the same side of the carbon-carbon bond.
Thus, only $ 1 $ is the product. Option a is the correct one.
Note:
When Osmium tetroxide is used with the combination of reagents other than trimethyl acetic acid anti addition may take place, and in some cases both the syn and anti-addition takes place. In that case, a racemic mixture will be formed. But, here only syn diols will be formed.
Complete answer:
Alkenes consist of double bonds, the general molecular formula of alkenes is $ {C_n}{H_{2n}} $ , where n is the number of carbon atoms. Due to the presence of a double bond, the carbon in alkenes has $ s{p^2} $ hybridisation. Alkenes participate in additional reactions due to the presence of double bonds.
Osmium tetroxide with the combination of hydrogen peroxide or tri methyl acetic acid used as a material for the synthesis of $ 1,2 $ diols from alkenes.
Given molecules is $ 2 - butene $ when treated with osmium tetroxide and tri methyl acetic acid, the syn addition of hydroxyl groups takes place. Thus, the diols formed will be syn-diols.
In the given reaction, only one product is syn diol. The syn diol has two hydroxyl groups on the same side of the carbon-carbon bond.
Thus, only $ 1 $ is the product. Option a is the correct one.
Note:
When Osmium tetroxide is used with the combination of reagents other than trimethyl acetic acid anti addition may take place, and in some cases both the syn and anti-addition takes place. In that case, a racemic mixture will be formed. But, here only syn diols will be formed.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




